Regulation of cardiac O-GlcNAcylation: More than just nutrient availability
- PMID: 32014551
- PMCID: PMC7703857
- DOI: 10.1016/j.bbadis.2020.165712
Regulation of cardiac O-GlcNAcylation: More than just nutrient availability
Abstract
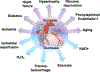
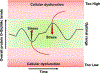
The post-translational modification of serine and threonine residues of nuclear, cytosolic, and mitochondrial proteins by O-linked β-N-acetyl glucosamine (O-GlcNAc) has long been seen as an important regulatory mechanism in the cardiovascular system. O-GlcNAcylation of cardiac proteins has been shown to contribute to the regulation of transcription, metabolism, mitochondrial function, protein quality control and turnover, autophagy, and calcium handling. In the heart, acute increases in O-GlcNAc have been associated with cardioprotection, such as those observed during ischemia/reperfusion. Conversely, chronic increases in O-GlcNAc, often associated with diabetes and nutrient excess, have been shown to contribute to cardiac dysfunction. Traditionally, many studies have linked changes in O-GlcNAc with nutrient availability and as such O-GlcNAcylation is often seen as a nutrient driven process. However, emerging evidence suggests that O-GlcNAcylation may also be regulated by non-nutrient dependent mechanisms, such as transcriptional and post-translational regulation. Therefore, the goals of this review are to provide an overview of the impact of O-GlcNAcylation in the cardiovascular system, how this is regulated and to discuss the emergence of regulatory mechanisms other than nutrient availability.
Keywords: Cardiomyocyte; GFAT; Heart; Metabolism; Nutrient regulation; O-GlcNAc; O-GlcNAc transferase (OGT); O-GlcNAcase (OGA).
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors do not have any conflicts of interest and/or disclosures to declare.
Figures

References
-
- Torres CR, Hart GW, Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc, The Journal of biological chemistry, 259 (1984) 3308–3317. - PubMed
-
- Zachara NE, Hart GW, O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress, Biochimica et biophysica acta, 1673 (2004) 13–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

