A peptide derived from the core β-sheet region of TIRAP decoys TLR4 and reduces inflammatory and autoimmune symptoms in murine models
- PMID: 32014819
- PMCID: PMC6997517
- DOI: 10.1016/j.ebiom.2020.102645
A peptide derived from the core β-sheet region of TIRAP decoys TLR4 and reduces inflammatory and autoimmune symptoms in murine models
Erratum in
-
Corrigendum to "A peptide derived from the core β-sheet region of TIRAP decoys TLR4 and reduces inflammatory and autoimmune symptoms in murine models" [eBioMedicine 52 (2020) 102645].EBioMedicine. 2025 Nov;121:105953. doi: 10.1016/j.ebiom.2025.105953. Epub 2025 Oct 29. EBioMedicine. 2025. PMID: 41166992 Free PMC article. No abstract available.
Abstract
Background: TLRs are some of the actively pursued drug-targets in immune disorders. Owing to a recent surge in the cognizance of TLR structural biology and signalling pathways, numerous therapeutic modulators, ranging from low-molecular-weight organic compounds to polypeptides and nucleic acid agents have been developed.
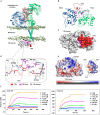
Methods: A penetratin-conjugated small peptide (TIP3), derived from the core β-sheet of TIRAP, was evaluated in vitro by monitoring the TLR-mediated cytokine induction and quantifying the protein expression using western blot. The therapeutic potential of TIP3 was further evaluated in TLR-dependent in vivo disease models.
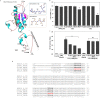
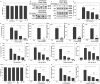
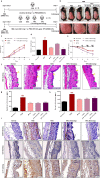
Findings: TIP3 blocks the TLR4-mediated cytokine production through both the MyD88- and TRIF-dependent pathways. A similar inhibitory-effect was exhibited for TLR3 but not on other TLRs. A profound therapeutic effect was observed in vivo, where TIP3 successfully alleviated the inflammatory response in mice model of collagen-induced arthritis and ameliorated the disease symptoms in psoriasis and SLE models.
Interpretation: Our data suggest that TIP3 may be a potential lead candidate for the development of effective therapeutics against TLR-mediated autoimmune disorders.
Funding: This work was supported by the National Research Foundation of Korea (NRF-2019M3A9A8065098, 2019M3D1A1078940 and 2019R1A6A1A11051471). The funders did not have any role in the design of the present study, data collection, data analysis, interpretation, or the writing of the manuscript.
Keywords: Antagonist; Collagen-induced arthritis; Decoy peptide; Psoriasis; Systemic lupus erythematosus; TLR4.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that there are no conflicts of interest.
Figures



References
-
- Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. - PubMed
-
- Gay N.J., Gangloff M., Weber A.N. Toll-like receptors as molecular switches. Nat Rev Immunol. 2006;6(9):693–698. - PubMed
-
- O'Neill L.A., Bowie A.G. The family of five: tIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7(5):353–364. - PubMed
-
- Gay N.J., Symmons M.F., Gangloff M., Bryant C.E. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol. 2014;14(8):546–558. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

