Expanding the phenotypic spectrum in RDH12-associated retinal disease
- PMID: 32014858
- PMCID: PMC6996522
- DOI: 10.1101/mcs.a004754
Expanding the phenotypic spectrum in RDH12-associated retinal disease
Abstract
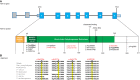
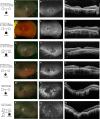
Retinol dehydrogenase 12, RDH12, plays a pivotal role in the visual cycle to ensure the maintenance of normal vision. Alterations in activity of this protein result in photoreceptor death and decreased vision beginning at an early age and progressing to substantial vision loss later in life. Here we describe 11 patients with retinal degeneration that underwent next-generation sequencing (NGS) with a targeted panel of all currently known inherited retinal degeneration (IRD) genes and whole-exome sequencing to identify the genetic causality of their retinal disease. These patients display a range of phenotypic severity prompting clinical diagnoses of macular dystrophy, cone-rod dystrophy, retinitis pigmentosa, and early-onset severe retinal dystrophy all attributed to biallelic recessive mutations in RDH12 We report 15 causal alleles and expand the repertoire of known RDH12 mutations with four novel variants: c.215A > G (p.Asp72Gly); c.362T > C (p.Ile121Thr); c.440A > C (p.Asn147Thr); and c.697G > A (p.Val233Ille). The broad phenotypic spectrum observed with biallelic RDH12 mutations has been observed in other genetic forms of IRDs, but the diversity is particularly notable here given the prior association of RDH12 primarily with severe early-onset disease. This breadth emphasizes the importance of broad genetic testing for inherited retinal disorders and extends the pool of individuals who may benefit from imminent gene-targeted therapies.
Keywords: central scotoma; cone-rod dystrophy; macular dystrophy; peripheral visual field loss; pigmentary retinal degeneration; progressive central visual loss; progressive visual field defects; severe visual impairment.
© 2020 Scott et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
References
-
- Albert S, Garanto A, Sangermano R, Khan M, Bax NM, Hoyng CB, Zernant J, Lee W, Allikmets R, Collin RWJ, et al. 2018. Identification and rescue of splice defects caused by two neighboring deep-intronic ABCA4 mutations underlying Stargardt disease. Am J Hum Genet 102: 517–527. 10.1016/j.ajhg.2018.02.008 - DOI - PMC - PubMed
-
- Aleman TS, Uyhazi KE, Serrano LW, Vasireddy V, Bowman SJ, Ammar MJ, Pearson DJ, Maguire AM, Bennett J. 2018. RDH12 mutations cause a severe retinal degeneration with relatively spared rod function retinal structure and function in RDH12-LCA. Invest Ophthalmol Vis Sci 59: 5225–5236. 10.1167/iovs.18-24708 - DOI - PubMed
-
- Badano JL, Kim JC, Hoskins BE, Lewis RA, Ansley SJ, Cutler DJ, Castellan C, Beales PL, Leroux MR, Katsanis N. 2003. Heterozygous mutations in BBS1, BBS2 and BBS6 have a potential epistatic effect on Bardet–Biedl patients with two mutations at a second BBS locus. Hum Mol Genet 12: 1651–1659. 10.1093/hmg/ddg188 - DOI - PubMed
-
- Bax NM, Sangermano R, Roosing S, Thiadens AA, Hoefsloot LH, van den Born LI, Phan M, Klevering BJ, Westeneng-van Haaften C, Braun TA, et al. 2015. Heterozygous deep-intronic variants and deletions in ABCA4 in persons with retinal dystrophies and one exonic ABCA4 variant. Hum Mutat 36: 43–47. 10.1002/humu.22717 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
