Dissemination of trial results to participants in phase III pragmatic clinical trials: an audit of trial investigators intentions
- PMID: 32014881
- PMCID: PMC7045144
- DOI: 10.1136/bmjopen-2019-035730
Dissemination of trial results to participants in phase III pragmatic clinical trials: an audit of trial investigators intentions
Abstract
Objective: To determine the proportion of Phase III clinical trials given a favourable opinion by a research ethics committee in the UK that provided trial results to those who participated.
Design: Audit of records.
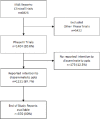
Setting: Phase III clinical trials registered on the UK's research permissions system (Integrated Research Application System) between the 1 January 2012 to 31 December 2017.
Main outcome measures: Proportion of trial investigators that intended to provide results to trial participants compared against what trials reported to ethics committees at the end of study.
Results: Out of 1404 Phase III trials, 87.7% (n=1231) trials stated they intended to disseminate results to participants while 12.3% (n=173) trials stated they would not. Out of these 1231 trials, 18.8% (n=231) trials intended to actively communicate trial results or a means of accessing results to their participants, a further 80.5% (n=991) reported passive intention to disseminate and for the remainder (n=9) the process was unclear. Of the 370 End of Study reports (30% of all included studies) that could be accessed 10 (2.7%) explicitly mentioned activities related to dissemination of findings to participants with the majority (74.9%) having no mention and a further 22.4% of reports not being accessible. Of the 10 which did report dissemination of results to participants the majority (n=6) were through a lay summary or letter.
Conclusions: Reported intention to disseminate results to trial participants among trial investigators is high, however, reporting of feedback methods is lacking. In addition, mechanisms to ensure intentions to disseminate trial results are translated into actual behaviour need to be put in place to ensure those who participate in trials have the opportunity to find out about the results.
Keywords: clinical audit; clinical trials; medical ethics.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: This audit forms part of a larger project that aims to develop recommendations for how to appropriately feedback trial results to those who participated in them. This overarching project is funded by the Academy of Medical Science (SBF002\1014: Chief Investigator KG and supported HB, RECAP: researchregistry4085). KG was supported by an MRC Methodology Research Fellowship (MR/L01193X/1).
References
-
- National Institute for health research CRN performance report 2018/2019. Available: https://www.nihr.ac.uk/documents/performance-report-year-end-201819/21871 [Accessed 07 Nov 2019].
-
- Research participant experience survey report 2018-19. Available: https://www.nihr.ac.uk/documents/research-participant-experience-survey-... [Accessed 07 Nov 2019].
-
- Health Research Authority Publishing your research findings, 2019. Available: https://www.hra.nhs.uk/planning-and-improving-research/research-planning... [Accessed 07 Nov 2019].
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources