Fosamprenavir with Ritonavir Pharmacokinetics during Pregnancy
- PMID: 32015036
- PMCID: PMC7179299
- DOI: 10.1128/AAC.02260-19
Fosamprenavir with Ritonavir Pharmacokinetics during Pregnancy
Abstract
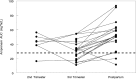
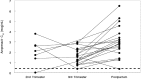
The purpose of this study was to evaluate the pharmacokinetics of ritonavir-boosted fosamprenavir during pregnancy and postpartum. Amprenavir (the active moiety of fosamprenavir) and ritonavir intensive pharmacokinetic evaluations were performed at steady state during the second and third trimesters of pregnancy and postpartum. Plasma concentrations of amprenavir and ritonavir were measured using high-performance liquid chromatography. The target amprenavir area under the concentration-versus-time curve (AUC) was higher than the 10th percentile (27.7 μg · h/ml) of the median area under the curve for ritonavir-boosted fosamprenavir in adults receiving twice-daily fosamprenavir-ritonavir at 700 mg/100 mg. Twenty-nine women were included in the analysis. The amprenavir AUC from time zero to 12 h (AUC0-12) was lower (geometric mean ratio [GMR], 0.60 [confidence interval {CI}, 0.49 to 0.72] [P < 0.001]) while its apparent oral clearance was higher (GMR, 1.68 [CI, 1.38 to 2.03] [P < 0.001]) in the third trimester than postpartum. Similarly, the ritonavir AUC0-12 was lower in the second (GMR, 0.51 [CI, 0.28 to 0.91] [P = 0.09]) and third (GMR, 0.72 [CI, 0.55 to 0.95] [P = 0.005]) trimesters than postpartum, while its apparent oral clearance was higher in the second (GMR, 1.98 [CI, 1.10 to 3.56] [P = 0.06]) and third (GMR, 1.38 [CI, 1.05 to 1.82] [P = 0.009]) trimesters than postpartum. The amprenavir area under the curve exceeded the target for 6/8 (75%) women in the 2nd trimester, 18/28 (64%) in the 3rd trimester, and 19/22 (86.4%) postpartum, and the trough concentrations (Cmin) of amprenavir were 4- to 16-fold above the mean amprenavir-protein-adjusted 50% inhibitory concentration (IC50) of 0.146 μg/ml. Although amprenavir plasma concentrations in women receiving ritonavir-boosted fosamprenavir were lower during pregnancy than postpartum, the reduced amprenavir concentrations were still above the exposures needed for viral suppression.
Keywords: AIDS; amprenavir; fosamprenavir; human immunodeficiency virus; pharmacokinetics; postpartum; pregnancy; ritonavir.
Copyright © 2020 American Society for Microbiology.
Figures
References
-
- Office of AIDS Research, NIH. 2018. Fosamprenavir (Lexiva, FPV). Office of AIDS Research, NIH, Bethesda, MD: https://aidsinfo.nih.gov/guidelines/html/3/perinatal/210/fosamprenavir-l.... Accessed 1 November 2019.
-
- Furfine ES, Baker CT, Hale MR, Reynolds DJ, Salisbury JA, Searle AD, Studenberg SD, Todd D, Tung RD, Spaltenstein A. 2004. Preclinical pharmacology and pharmacokinetics of GW433908, a water-soluble prodrug of the human immunodeficiency virus protease inhibitor amprenavir. Antimicrob Agents Chemother 48:791–798. doi:10.1128/aac.48.3.791-798.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

