Enhanced Lipid Accumulation and Metabolism Are Required for the Differentiation and Activation of Tumor-Associated Macrophages
- PMID: 32015091
- PMCID: PMC7127942
- DOI: 10.1158/0008-5472.CAN-19-2994
Enhanced Lipid Accumulation and Metabolism Are Required for the Differentiation and Activation of Tumor-Associated Macrophages
Erratum in
-
Correction: Enhanced Lipid Accumulation and Metabolism Are Required for the Differentiation and Activation of Tumor-Associated Macrophages.Cancer Res. 2022 Mar 1;82(5):945. doi: 10.1158/0008-5472.CAN-21-4296. Cancer Res. 2022. PMID: 35247899 No abstract available.
Abstract
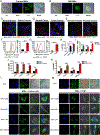
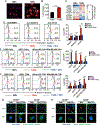
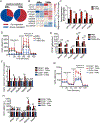
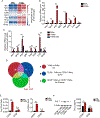
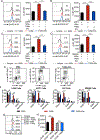
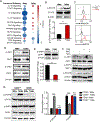
Tumor-associated macrophages (TAM) are important tumor-promoting cells. However, the mechanisms underlying how the tumor and its microenvironment reprogram these cells remain elusive. Here we report that lipids play a crucial role in generating TAMs in the tumor microenvironment (TME). Macrophages from both human and murine tumor tissues were enriched with lipids due to increased lipid uptake by macrophages. TAMs expressed elevated levels of the scavenger receptor CD36, accumulated lipids, and used fatty acid oxidation (FAO) instead of glycolysis for energy. High levels of FAO promoted mitochondrial oxidative phosphorylation, production of reactive oxygen species, phosphorylation of JAK1, and dephosphorylation of SHP1, leading to STAT6 activation and transcription of genes that regulate TAM generation and function. These processes were critical for TAM polarization and activity, both in vitro and in vivo. In summary, we highlight the importance of lipid metabolism in the differentiation and function of protumor TAMs in the TME. SIGNIFICANCE: This study highlights the role of lipid metabolism in the differentiation and function of TAMs and suggests targeting TAM fatty acid oxidation as a potential therapeutic modality for human cancers.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures

References
-
- Yang C, He L, He P, Liu Y, Wang W, He Y, et al. Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Med Oncol 2015;32:352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

