Combining microenvironment normalization strategies to improve cancer immunotherapy
- PMID: 32015113
- PMCID: PMC7035612
- DOI: 10.1073/pnas.1919764117
Combining microenvironment normalization strategies to improve cancer immunotherapy
Abstract
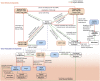
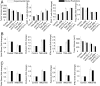
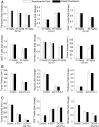
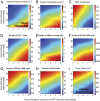
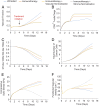
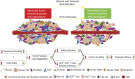
Advances in immunotherapy have revolutionized the treatment of multiple cancers. Unfortunately, tumors usually have impaired blood perfusion, which limits the delivery of therapeutics and cytotoxic immune cells to tumors and also results in hypoxia-a hallmark of the abnormal tumor microenvironment (TME)-that causes immunosuppression. We proposed that normalization of TME using antiangiogenic drugs and/or mechanotherapeutics can overcome these challenges. Recently, immunotherapy with checkpoint blockers was shown to effectively induce vascular normalization in some types of cancer. Although these therapeutic approaches have been used in combination in preclinical and clinical studies, their combined effects on TME are not fully understood. To identify strategies for improved immunotherapy, we have developed a mathematical framework that incorporates complex interactions among various types of cancer cells, immune cells, stroma, angiogenic molecules, and the vasculature. Model predictions were compared with the data from five previously reported experimental studies. We found that low doses of antiangiogenic treatment improve immunotherapy when the two treatments are administered sequentially, but that high doses are less efficacious because of excessive vessel pruning and hypoxia. Stroma normalization can further increase the efficacy of immunotherapy, and the benefit is additive when combined with vascular normalization. We conclude that vessel functionality dictates the efficacy of immunotherapy, and thus increased tumor perfusion should be investigated as a predictive biomarker of response to immunotherapy.
Keywords: anti-angiogenic therapy; immunotherapy; mechanotherapeutics; normalization; vascular function.
Conflict of interest statement
Competing interest statement: R.K.J. has received honoraria from Amgen and consultant fees from Chugai, Merck, Ophthotech, Pfizer, SPARC, SynDevRx, and XTuit; owns equity in Enlight, Ophthotech, and SynDevRx; and serves on the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, and Tekla World Healthcare Fund. D.G.D. received consultant fees from Bayer, Simcere, and BMS and research grants from Bayer, Exelixis, and BMS. Neither any reagent nor any funding from these organizations was used in this study.
Figures

Comment in
-
Immunoactivating the tumor microenvironment enhances immunotherapy as predicted by integrative computational model.Proc Natl Acad Sci U S A. 2020 Mar 3;117(9):4447-4449. doi: 10.1073/pnas.2001050117. Epub 2020 Feb 26. Proc Natl Acad Sci U S A. 2020. PMID: 32102915 Free PMC article. No abstract available.
Similar articles
-
Modulating cancer mechanopathology to restore vascular function and enhance immunotherapy.Cell Rep Med. 2024 Jul 16;5(7):101626. doi: 10.1016/j.xcrm.2024.101626. Epub 2024 Jun 28. Cell Rep Med. 2024. PMID: 38944037 Free PMC article. Review.
-
Targeting vascular normalization: a promising strategy to improve immune-vascular crosstalk in cancer immunotherapy.Front Immunol. 2023 Dec 15;14:1291530. doi: 10.3389/fimmu.2023.1291530. eCollection 2023. Front Immunol. 2023. PMID: 38193080 Free PMC article. Review.
-
TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity.Theranostics. 2020 Jan 12;10(4):1910-1922. doi: 10.7150/thno.36936. eCollection 2020. Theranostics. 2020. PMID: 32042344 Free PMC article.
-
Normalization of tumor vasculature: A potential strategy to increase the efficiency of immune checkpoint blockades in cancers.Int Immunopharmacol. 2022 Sep;110:108968. doi: 10.1016/j.intimp.2022.108968. Epub 2022 Jun 25. Int Immunopharmacol. 2022. PMID: 35764018 Review.
-
Manipulation of the crosstalk between tumor angiogenesis and immunosuppression in the tumor microenvironment: Insight into the combination therapy of anti-angiogenesis and immune checkpoint blockade.Front Immunol. 2022 Nov 10;13:1035323. doi: 10.3389/fimmu.2022.1035323. eCollection 2022. Front Immunol. 2022. PMID: 36439137 Free PMC article. Review.
Cited by
-
Mechanistic Target of Rapamycin Inhibitors in Renal Cell Carcinoma: Potential, Limitations, and Perspectives.Front Cell Dev Biol. 2021 Mar 15;9:636037. doi: 10.3389/fcell.2021.636037. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33791295 Free PMC article. Review.
-
Understanding the Crosstalk Between Epigenetics and Immunometabolism to Combat Cancer.Subcell Biochem. 2022;100:581-616. doi: 10.1007/978-3-031-07634-3_18. Subcell Biochem. 2022. PMID: 36301507
-
Quantitative multiplex immunohistochemistry reveals inter-patient lymphovascular and immune heterogeneity in primary cutaneous melanoma.Front Immunol. 2024 Feb 1;15:1328602. doi: 10.3389/fimmu.2024.1328602. eCollection 2024. Front Immunol. 2024. PMID: 38361951 Free PMC article.
-
The Role of Imaging Biomarkers to Guide Pharmacological Interventions Targeting Tumor Hypoxia.Front Pharmacol. 2022 Jul 15;13:853568. doi: 10.3389/fphar.2022.853568. eCollection 2022. Front Pharmacol. 2022. PMID: 35910347 Free PMC article. Review.
-
Tumor vessel normalization and immunotherapy in gastric cancer.Ther Adv Med Oncol. 2022 Jul 18;14:17588359221110176. doi: 10.1177/17588359221110176. eCollection 2022. Ther Adv Med Oncol. 2022. PMID: 35872968 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

