Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling
- PMID: 32015118
- PMCID: PMC7035606
- DOI: 10.1073/pnas.1912130117
Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling
Abstract
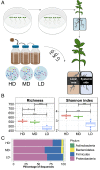
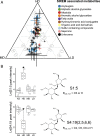
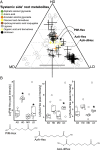
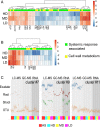
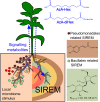
Microbial communities associated with roots confer specific functions to their hosts, thereby modulating plant growth, health, and productivity. Yet, seminal questions remain largely unaddressed including whether and how the rhizosphere microbiome modulates root metabolism and exudation and, consequently, how plants fine tune this complex belowground web of interactions. Here we show that, through a process termed systemically induced root exudation of metabolites (SIREM), different microbial communities induce specific systemic changes in tomato root exudation. For instance, systemic exudation of acylsugars secondary metabolites is triggered by local colonization of bacteria affiliated with the genus Bacillus Moreover, both leaf and systemic root metabolomes and transcriptomes change according to the rhizosphere microbial community structure. Analysis of the systemic root metabolome points to glycosylated azelaic acid as a potential microbiome-induced signaling molecule that is subsequently exuded as free azelaic acid. Our results demonstrate that rhizosphere microbiome assembly drives the SIREM process at the molecular and chemical levels. It highlights a thus-far unexplored long-distance signaling phenomenon that may regulate soil conditioning.
Keywords: long-distance signaling; metabolomics; microbiome; root exudation.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
Volatile-mediated interspecific plant interaction promotes root colonization by beneficial bacteria via induced shifts in root exudation.Microbiome. 2024 Oct 21;12(1):207. doi: 10.1186/s40168-024-01914-w. Microbiome. 2024. PMID: 39428455 Free PMC article.
-
Cover crop root exudates impact soil microbiome functional trajectories in agricultural soils.Microbiome. 2024 Sep 28;12(1):183. doi: 10.1186/s40168-024-01886-x. Microbiome. 2024. PMID: 39342284 Free PMC article.
-
Fusaric acid mediates the assembly of disease-suppressive rhizosphere microbiota via induced shifts in plant root exudates.Nat Commun. 2024 Jun 15;15(1):5125. doi: 10.1038/s41467-024-49218-9. Nat Commun. 2024. PMID: 38879580 Free PMC article.
-
Root exudates: from plant to rhizosphere and beyond.Plant Cell Rep. 2020 Jan;39(1):3-17. doi: 10.1007/s00299-019-02447-5. Epub 2019 Jul 25. Plant Cell Rep. 2020. PMID: 31346716 Review.
-
Root exudates and microbial metabolites: signals and nutrients in plant-microbe interactions.Sci China Life Sci. 2025 Aug;68(8):2290-2302. doi: 10.1007/s11427-024-2876-0. Epub 2025 Mar 11. Sci China Life Sci. 2025. PMID: 40080268 Review.
Cited by
-
Root phenotypes as modulators of microbial microhabitats.Front Plant Sci. 2022 Sep 23;13:1003868. doi: 10.3389/fpls.2022.1003868. eCollection 2022. Front Plant Sci. 2022. PMID: 36212354 Free PMC article. Review.
-
Variation in Root Exudate Composition Influences Soil Microbiome Membership and Function.Appl Environ Microbiol. 2022 Jun 14;88(11):e0022622. doi: 10.1128/aem.00226-22. Epub 2022 May 10. Appl Environ Microbiol. 2022. PMID: 35536051 Free PMC article.
-
Microbiome of Field Grown Hemp Reveals Potential Microbial Interactions With Root and Rhizosphere Soil.Front Microbiol. 2021 Nov 15;12:741597. doi: 10.3389/fmicb.2021.741597. eCollection 2021. Front Microbiol. 2021. PMID: 34867858 Free PMC article.
-
Identifying plant genes shaping microbiota composition in the barley rhizosphere.Nat Commun. 2022 Jun 16;13(1):3443. doi: 10.1038/s41467-022-31022-y. Nat Commun. 2022. PMID: 35710760 Free PMC article.
-
Chemically Mediated Plant-Plant Interactions: Allelopathy and Allelobiosis.Plants (Basel). 2024 Feb 24;13(5):626. doi: 10.3390/plants13050626. Plants (Basel). 2024. PMID: 38475470 Free PMC article. Review.
References
-
- Herrera Paredes S., Lebeis S. L., Giving back to the community: Microbial mechanisms of plant–soil interactions. Funct. Ecol. 30, 1043–1052 (2016).
-
- Ofek-Lalzar M., et al. , Niche and host-associated functional signatures of the root surface microbiome. Nat. Commun. 5, 4950 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

