M2 amphipathic helices facilitate pH-dependent conformational transition in influenza A virus
- PMID: 32015120
- PMCID: PMC7035486
- DOI: 10.1073/pnas.1913385117
M2 amphipathic helices facilitate pH-dependent conformational transition in influenza A virus
Abstract
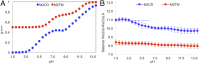
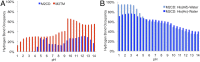
The matrix-2 (M2) protein from influenza A virus is a tetrameric, integral transmembrane (TM) protein that plays a vital role in viral replication by proton flux into the virus. The His37 tetrad is a pH sensor in the center of the M2 TM helix that activates the channel in response to the low endosomal pH. M2 consists of different regions that are believed to be involved in membrane targeting, packaging, nucleocapsid binding, and proton transport. Although M2 has been the target of many experimental and theoretical studies that have led to significant insights into its structure and function under differing conditions, the main mechanism of proton transport, its conformational dynamics, and the role of the amphipathic helices (AHs) on proton conductance remain elusive. To this end, we have applied explicit solvent constant pH molecular dynamics using the multisite λ-dynamics approach (CpHMDMSλD) to investigate the buried ionizable residues comprehensively and to elucidate their effect on the conformational transition. Our model recapitulates the pH-dependent conformational transition of M2 from closed to open state when the AH domain is included in the M2 construct, revealing the role of the amphipathic helices on this transition and shedding light on the proton-transport mechanism. This work demonstrates the importance of including the amphipathic helices in future experimental and theoretical studies of ion channels. Finally, our work shows that explicit solvent CpHMDMSλD provides a realistic pH-dependent model for membrane proteins.
Keywords: M2 transmembrane protein; amphipathic helix; explicit solvent CpHMD.
Conflict of interest statement
The authors declare no competing interest.
Figures
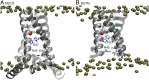


Similar articles
-
Structure and mechanism of the M2 proton channel of influenza A virus.Nature. 2008 Jan 31;451(7178):591-5. doi: 10.1038/nature06531. Nature. 2008. PMID: 18235503 Free PMC article.
-
Structural Transition from Closed to Open for the Influenza A M2 Proton Channel as Observed by Proton-Detected Solid-State NMR.J Am Chem Soc. 2025 Aug 6;147(31):27537-27551. doi: 10.1021/jacs.5c05111. Epub 2025 Jun 20. J Am Chem Soc. 2025. PMID: 40539990 Free PMC article.
-
Structure and mechanism of proton transport through the transmembrane tetrameric M2 protein bundle of the influenza A virus.Proc Natl Acad Sci U S A. 2010 Aug 24;107(34):15075-80. doi: 10.1073/pnas.1007071107. Epub 2010 Aug 5. Proc Natl Acad Sci U S A. 2010. PMID: 20689043 Free PMC article.
-
M2 protein from influenza A: from multiple structures to biophysical and functional insights.Curr Opin Virol. 2012 Apr;2(2):128-33. doi: 10.1016/j.coviro.2012.01.005. Epub 2012 Feb 16. Curr Opin Virol. 2012. PMID: 22482709 Free PMC article. Review.
-
Structural and dynamic mechanisms for the function and inhibition of the M2 proton channel from influenza A virus.Curr Opin Struct Biol. 2011 Feb;21(1):68-80. doi: 10.1016/j.sbi.2010.12.002. Epub 2011 Jan 17. Curr Opin Struct Biol. 2011. PMID: 21247754 Free PMC article. Review.
Cited by
-
From Acid Activation Mechanisms of Proton Conduction to Design of Inhibitors of the M2 Proton Channel of Influenza A Virus.Front Mol Biosci. 2022 Jan 14;8:796229. doi: 10.3389/fmolb.2021.796229. eCollection 2021. Front Mol Biosci. 2022. PMID: 35096969 Free PMC article. Review.
-
Real-time tracking of drug binding to influenza A M2 reveals a high energy barrier.J Struct Biol X. 2023 Jun 7;8:100090. doi: 10.1016/j.yjsbx.2023.100090. eCollection 2023 Dec. J Struct Biol X. 2023. PMID: 37363040 Free PMC article.
-
Activation of the influenza B M2 proton channel (BM2).bioRxiv [Preprint]. 2024 Jul 26:2024.07.26.605324. doi: 10.1101/2024.07.26.605324. bioRxiv. 2024. Update in: Biochemistry. 2024 Nov 19;63(22):3011-3019. doi: 10.1021/acs.biochem.4c00607. PMID: 39091734 Free PMC article. Updated. Preprint.
-
Cholesterol and M2 Rendezvous in Budding and Scission of Influenza A Virus.Subcell Biochem. 2023;106:441-459. doi: 10.1007/978-3-031-40086-5_16. Subcell Biochem. 2023. PMID: 38159237
-
YWHAG inhibits influenza a virus replication by suppressing the release of viral M2 protein.Front Microbiol. 2022 Jul 19;13:951009. doi: 10.3389/fmicb.2022.951009. eCollection 2022. Front Microbiol. 2022. PMID: 35928168 Free PMC article.
References
-
- Pinto L. H., Holsinger L. J., Lamb R. A., Influenza virus M2 protein has ion channel activity. Cell 69, 517–528 (1992). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

