High-throughput quantitative microscopy-based half-life measurements of intravenously injected agents
- PMID: 32015123
- PMCID: PMC7035491
- DOI: 10.1073/pnas.1915450117
High-throughput quantitative microscopy-based half-life measurements of intravenously injected agents
Abstract
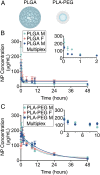
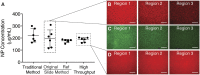
Accurate analysis of blood concentration and circulation half-life is an important consideration for any intravenously administered agent in preclinical development or for therapeutic application. However, the currently available tools to measure these parameters are laborious, expensive, and inefficient for handling multiple samples from complex multivariable experiments. Here we describe a robust high-throughput quantitative microscopy-based method to measure the blood concentration and circulation half-life of any fluorescently labeled agent using only a small (2 µL) amount of blood volume, enabling additional end-point measurements to be assessed in the same subject. To validate this method, we demonstrate its use to measure the circulation half-life in mice of two types of fluorescently labeled polymeric nanoparticles of different sizes and surface chemistries and of a much smaller fluorescently labeled monoclonal antibody. Furthermore, we demonstrate the improved accuracy of this method compared to previously described methods.
Keywords: circulation half-life; drug delivery; nanoparticle; quantitative microscopy.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Quantitative microscopy-based measurements of circulating nanoparticle concentration using microliter blood volumes.Nanomedicine. 2017 Aug;13(6):1863-1867. doi: 10.1016/j.nano.2017.04.003. Epub 2017 Apr 13. Nanomedicine. 2017. PMID: 28412144 Free PMC article.
-
The development and assessment of high-throughput mass spectrometry-based methods for the quantification of a nanoparticle drug delivery agent in cellular lysate.J Mass Spectrom. 2014 Nov;49(11):1171-80. doi: 10.1002/jms.3444. J Mass Spectrom. 2014. PMID: 25395133
-
Suspensions for intravenous (IV) injection: a review of development, preclinical and clinical aspects.Adv Drug Deliv Rev. 2008 May 22;60(8):939-54. doi: 10.1016/j.addr.2007.11.008. Epub 2008 Feb 7. Adv Drug Deliv Rev. 2008. PMID: 18343527 Review.
-
Addressing the challenges of low clearance in drug research.AAPS J. 2015 Mar;17(2):352-7. doi: 10.1208/s12248-014-9691-7. Epub 2015 Jan 8. AAPS J. 2015. PMID: 25567366 Free PMC article. Review.
-
Quantification of DOX bioavailability in biological samples of mice by sensitive and precise HPLC assay.Pharm Biol. 2016;54(1):55-61. doi: 10.3109/13880209.2015.1014918. Epub 2015 Apr 16. Pharm Biol. 2016. PMID: 25880143
Cited by
-
Cellular determinants influence the red blood cell adsorption efficiency of poly(amine-co-ester) nanoparticles.Sci Adv. 2025 May 2;11(18):eadt8637. doi: 10.1126/sciadv.adt8637. Epub 2025 May 2. Sci Adv. 2025. PMID: 40315323 Free PMC article.
-
Investigation of the protein corona and biodistribution profile of polymeric nanoparticles for intra-amniotic delivery.Biomaterials. 2025 Sep;320:123238. doi: 10.1016/j.biomaterials.2025.123238. Epub 2025 Mar 6. Biomaterials. 2025. PMID: 40064138
-
Modifying the Backbone Chemistry of PEG-Based Bottlebrush Block Copolymers for the Formation of Long-Circulating Nanoparticles.Adv Healthc Mater. 2024 Sep;13(22):e2304040. doi: 10.1002/adhm.202304040. Epub 2024 May 22. Adv Healthc Mater. 2024. PMID: 38734871
-
PEGylation of poly(amine-co-ester) polyplexes for tunable gene delivery.Biomaterials. 2021 May;272:120780. doi: 10.1016/j.biomaterials.2021.120780. Epub 2021 Mar 24. Biomaterials. 2021. PMID: 33813260 Free PMC article.
-
Enhancing in vivo cell and tissue targeting by modulation of polymer nanoparticles and macrophage decoys.Nat Commun. 2024 May 18;15(1):4247. doi: 10.1038/s41467-024-48442-7. Nat Commun. 2024. PMID: 38762483 Free PMC article.
References
-
- Gillies S. D., et al. , Improved circulating half-life and efficacy of an antibody-interleukin 2 immunocytokine based on reduced intracellular proteolysis. Clin. Cancer Res. 8, 210–216 (2002). - PubMed
-
- Elgundi Z., Reslan M., Cruz E., Sifniotis V., Kayser V., The state-of-play and future of antibody therapeutics. Adv. Drug Deliv. Rev. 122, 2–19 (2017). - PubMed
-
- Ayyar B. V., Arora S., O’Kennedy R., Coming-of-age of antibodies in cancer therapeutics. Trends Pharmacol. Sci. 37, 1009–1028 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

