Protection of cochlear synapses from noise-induced excitotoxic trauma by blockade of Ca2+-permeable AMPA receptors
- PMID: 32015128
- PMCID: PMC7035499
- DOI: 10.1073/pnas.1914247117
Protection of cochlear synapses from noise-induced excitotoxic trauma by blockade of Ca2+-permeable AMPA receptors
Abstract
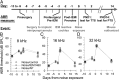
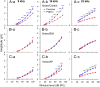
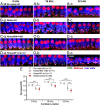
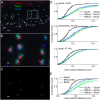
Exposure to loud sound damages the postsynaptic terminals of spiral ganglion neurons (SGNs) on cochlear inner hair cells (IHCs), resulting in loss of synapses, a process termed synaptopathy. Glutamatergic neurotransmission via α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)-type receptors is required for synaptopathy, and here we identify a possible involvement of GluA2-lacking Ca2+-permeable AMPA receptors (CP-AMPARs) using IEM-1460, which has been shown to block GluA2-lacking AMPARs. In CBA/CaJ mice, a 2-h exposure to 100-dB sound pressure level octave band (8 to 16 kHz) noise results in no permanent threshold shift but does cause significant synaptopathy and a reduction in auditory brainstem response (ABR) wave-I amplitude. Chronic intracochlear perfusion of IEM-1460 in artificial perilymph (AP) into adult CBA/CaJ mice prevented the decrease in ABR wave-I amplitude and the synaptopathy relative to intracochlear perfusion of AP alone. Interestingly, IEM-1460 itself did not affect the ABR threshold, presumably because GluA2-containing AMPARs can sustain sufficient synaptic transmission to evoke low-threshold responses during blockade of GluA2-lacking AMPARs. On individual postsynaptic densities, we observed GluA2-lacking nanodomains alongside regions with robust GluA2 expression, consistent with the idea that individual synapses have both CP-AMPARs and Ca2+-impermeable AMPARs. SGNs innervating the same IHC differ in their relative vulnerability to noise. We found local heterogeneity among synapses in the relative abundance of GluA2 subunits that may underlie such differences in vulnerability. We propose a role for GluA2-lacking CP-AMPARs in noise-induced cochlear synaptopathy whereby differences among synapses account for differences in excitotoxic susceptibility. These data suggest a means of maintaining normal hearing thresholds while protecting against noise-induced synaptopathy, via selective blockade of CP-AMPARs.
Keywords: Ca2+-permeable AMPA receptor; cochlear synapse; excitotoxicity; noise-induced synaptopathy; spiral ganglion neuron.
Conflict of interest statement
Competing interest statement: A provisional patent application titled “Targeting Calcium-Permeable AMPA Receptors for Inner Ear Therapy with IEM-1460 and Related Compounds” was filed on 23 December 2019. Some data presented in this paper was cited in the application.
Figures



References
-
- Safieddine S., El-Amraoui A., Petit C., The auditory hair cell ribbon synapse: From assembly to function. Annu. Rev. Neurosci. 35, 509–528 (2012). - PubMed
-
- Rutherford M. A., Moser T., “The ribbon synapse between type I spiral ganglion neurons and inner hair cells” in The Primary Auditory Neurons of the Mammalian Cochlea, Dabdoub A., Fritzsch B., Popper A. N., Fay R. R., Eds. (Springer, New York, 2016), pp. 117–156.
-
- Pujol R., Lenoir M., Robertson D., Eybalin M., Johnstone B. M., Kainic acid selectively alters auditory dendrites connected with cochlear inner hair cells. Hear. Res. 18, 145–151 (1985). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

