Basal-Level Effects of (p)ppGpp in the Absence of Branched-Chain Amino Acids in Actinobacillus pleuropneumoniae
- PMID: 32015147
- PMCID: PMC7099145
- DOI: 10.1128/JB.00640-19
Basal-Level Effects of (p)ppGpp in the Absence of Branched-Chain Amino Acids in Actinobacillus pleuropneumoniae
Abstract
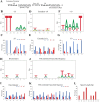
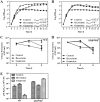
The (p)ppGpp-mediated stringent response (SR) is a highly conserved regulatory mechanism in bacterial pathogens, enabling adaptation to adverse environments, and is linked to pathogenesis. Actinobacillus pleuropneumoniae can cause damage to the lungs of pigs, its only known natural host. Pig lungs are known to have a low concentration of free branched-chain amino acids (BCAAs) compared to the level in plasma. We had investigated the role for (p)ppGpp in viability and biofilm formation of A. pleuropneumoniae Now, we sought to determine whether (p)ppGpp was a trigger signal for the SR in A. pleuropneumoniae in the absence of BCAAs. Combining transcriptome and phenotypic analyses of the wild type (WT) and an relA spoT double mutant [which does not produce (p)ppGpp], we found that (p)ppGpp could repress de novo purine biosynthesis and activate antioxidant pathways. There was a positive correlation between GTP and endogenous hydrogen peroxide content. Furthermore, the growth, viability, morphology, and virulence were altered by the inability to produce (p)ppGpp. Genes involved in the biosynthesis of BCAAs were constitutively upregulated, regardless of the existence of BCAAs, without accumulation of (p)ppGpp beyond a basal level. Collectively, our study shows that the absence of BCAAs was not a sufficient signal to trigger the SR in A. pleuropneumoniae (p)ppGpp-mediated regulation in A. pleuropneumoniae is different from that described for the model organism Escherichia coli Further work will establish whether the (p)ppGpp-dependent SR mechanism in A. pleuropneumoniae is conserved among other veterinary pathogens, especially those in the Pasteurellaceae family.IMPORTANCE (p)ppGpp is a key player in reprogramming transcriptomes to respond to nutritional challenges. Here, we present transcriptional and phenotypic differences of A. pleuropneumoniae grown in different chemically defined media in the absence of (p)ppGpp. We show that the deprivation of branched-chain amino acids (BCAAs) does not elicit a change in the basal-level (p)ppGpp, but this level is sufficient to regulate the expression of BCAA biosynthesis. The mechanism found in A. pleuropneumoniae is different from that of the model organism Escherichia coli but similar to that found in some Gram-positive bacteria. This study not only broadens the research scope of (p)ppGpp but also further validates the complexity and multiplicity of (p)ppGpp regulation in microorganisms that occupy different biological niches.
Keywords: (p)ppGpp; Actinobacillus pleuropneumoniae; BCAAs; GTP; stringent response.
Copyright © 2020 American Society for Microbiology.
Figures
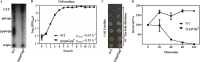





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

