Ferric Citrate Regulator FecR Is Translocated across the Bacterial Inner Membrane via a Unique Twin-Arginine Transport-Dependent Mechanism
- PMID: 32015149
- PMCID: PMC7148137
- DOI: 10.1128/JB.00541-19
Ferric Citrate Regulator FecR Is Translocated across the Bacterial Inner Membrane via a Unique Twin-Arginine Transport-Dependent Mechanism
Abstract
In Escherichia coli, citrate-mediated iron transport is a key nonheme pathway for the acquisition of iron. Binding of ferric citrate to the outer membrane protein FecA induces a signal cascade that ultimately activates the cytoplasmic sigma factor FecI, resulting in transcription of the fecABCDE ferric citrate transport genes. Central to this process is signal transduction mediated by the inner membrane protein FecR. FecR spans the inner membrane through a single transmembrane helix, which is flanked by cytoplasm- and periplasm-orientated moieties at the N and C termini. The transmembrane helix of FecR resembles a twin-arginine signal sequence, and the substitution of the paired arginine residues of the consensus motif decouples the FecR-FecI signal cascade, rendering the cells unable to activate transcription of the fec operon when grown on ferric citrate. Furthermore, the fusion of beta-lactamase C-terminal to the FecR transmembrane helix results in translocation of the C-terminal domain that is dependent on the twin-arginine translocation (Tat) system. Our findings demonstrate that FecR belongs to a select group of bitopic inner membrane proteins that contain an internal twin-arginine signal sequence.IMPORTANCE Iron is essential for nearly all living organisms due to its role in metabolic processes and as a cofactor for many enzymes. The FecRI signal transduction pathway regulates citrate-mediated iron import in many Gram-negative bacteria, including Escherichia coli The interactions of FecR with the outer membrane protein FecA and cytoplasmic anti-sigma factor FecI have been extensively studied. However, the mechanism by which FecR inserts into the membrane has not previously been reported. In this study, we demonstrate that the targeting of FecR to the cytoplasmic membrane is dependent on the Tat system. As such, FecR represents a new class of bitopic Tat-dependent membrane proteins with an internal twin-arginine signal sequence.
Keywords: Escherichia coli; iron acquisition; iron regulation; twin-arginine translocation.
Copyright © 2020 American Society for Microbiology.
Figures

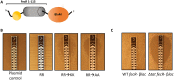
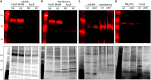
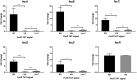
Comment in
- https://doi.org/10.1128/JB.00058-20 doi: 10.1128/JB.00058-20
References
-
- Staudenmaier H, Van Hove B, Yaraghi Z, Braun V. 1989. Nucleotide sequences of the fecBCDE genes and locations of the proteins suggest a periplasmic-binding-protein-dependent transport mechanism for iron(III) dicitrate in Escherichia coli. J Bacteriol 171:2626–2633. doi: 10.1128/jb.171.5.2626-2633.1989. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

