Handheld instrument for wound-conformal delivery of skin precursor sheets improves healing in full-thickness burns
- PMID: 32015225
- PMCID: PMC7042907
- DOI: 10.1088/1758-5090/ab6413
Handheld instrument for wound-conformal delivery of skin precursor sheets improves healing in full-thickness burns
Abstract
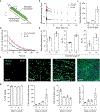
The current standard of care for patients with severe large-area burns consists of autologous skin grafting or acellular dermal substitutes. While emerging options to accelerate wound healing involve treatment with allogeneic or autologous cells, delivering cells to clinically relevant wound topologies, orientations, and sizes remains a challenge. Here, we report the one-step in situ formation of cell-containing biomaterial sheets using a handheld instrument that accommodates the topography of the wound. In an approach that maintained cell viability and proliferation, we demonstrated conformal delivery to surfaces that were inclined up to 45° with respect to the horizontal. In porcine pre-clinical models of full-thickness burn, we delivered mesenchymal stem/stromal cell-containing fibrin sheets directly to the wound bed, improving re-epithelialization, dermal cell repopulation, and neovascularization, indicating that this device could be introduced in a clinical setting improving dermal and epidermal regeneration.
Figures



References
-
- Greenhalgh D G, Saffle J R, Holmes J H, Gamelli R L, Palmieri T L, Horton J W, Tompkins R G, Traber D L, Mozingo D W, Deitch E A, Goodwin C W, Herndon D N, Gallagher J J, Sanford A P, Jeng J C, Ahrenholz D H, Neely A N, O’Mara M S, Wolf S E, Purdue G F, Garner W L, Yowler C J and Latenser B A 2007. American burn association consensus conference to define sepsis and infection in burns Journal of Burn Care and Research vol 28 pp 776–90 - PubMed
-
- Galeiras R, Lorente J A, Pértega S, Vallejo A, Tomicic V, de la Cal M A, Pita S, Cerdá E and Esteban A 2009. A model for predicting mortality among critically ill burn victims Burns 35 201–9 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
