Glenohumeral Osteoarthritis: The Role for Orthobiologic Therapies: Platelet-Rich Plasma and Cell Therapies
- PMID: 32015271
- PMCID: PMC7055935
- DOI: 10.2106/JBJS.RVW.19.00075
Glenohumeral Osteoarthritis: The Role for Orthobiologic Therapies: Platelet-Rich Plasma and Cell Therapies
Abstract
The glenohumeral (GH) joint ranks third on the list of the large joints that are most commonly affected by osteoarthritis, after the knee and the hip. General nonsurgical modalities, including changes in daily activities, physical therapy, pharmacotherapy, and corticosteroid injections, constitute the mainstay of treatment. Most of these options, however, have shown moderate and short-term effectiveness. Arthroplasty techniques have proven to be successful for elderly patients. Nevertheless, replacement options are not optimal for younger patients because their functional demands are higher and prostheses have a finite life span. This has led to the search for new nonoperative treatment options to target this subgroup of patients. It has been suggested that orthobiologic therapies, including platelet-rich plasma (PRP) and cell therapies, present great promise and opportunity for the treatment of GH osteoarthritis. Despite the promising results that have been shown by cell therapies and PRP for treating degenerative joint conditions, additional studies are needed to provide more definitive conclusions.
Figures
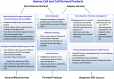
References
-
- Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ. Osteoarthritis. Lancet. 2015. July 25;386(9991):376-87. Epub 2015 Mar 4. - PubMed
-
- Leyland KM, Hart DJ, Javaid MK, Judge A, Kiran A, Soni A, Goulston LM, Cooper C, Spector TD, Arden NK. The natural history of radiographic knee osteoarthritis: a fourteen-year population-based cohort study. Arthritis Rheum. 2012. July;64(7):2243-51. - PubMed
-
- Saltzman BM, Leroux TS, Verma NN, Romeo AA. Glenohumeral osteoarthritis in the young patient. J Am Acad Orthop Surg. 2018. September 1;26(17):e361-70. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

