PABPC1-induced stabilization of BDNF-AS inhibits malignant progression of glioblastoma cells through STAU1-mediated decay
- PMID: 32015336
- PMCID: PMC6997171
- DOI: 10.1038/s41419-020-2267-9
PABPC1-induced stabilization of BDNF-AS inhibits malignant progression of glioblastoma cells through STAU1-mediated decay
Abstract
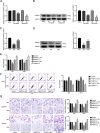
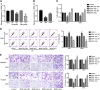
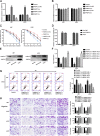
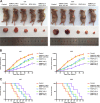
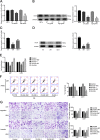
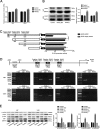
Glioblastoma is the most common and malignant form of primary central nervous tumor in adults. Long noncoding RNAs (lncRNAs) have been reported to play a pivotal role in modulating gene expression and regulating human tumor's malignant behaviors. In this study, we confirmed that lncRNA brain-derived neurotrophic factor antisense (BDNF-AS) was downregulated in glioblastoma tissues and cells, interacted and stabilized by polyadenylate-binding protein cytoplasmic 1 (PABPC1). Overexpression of BDNF-AS inhibited the proliferation, migration, and invasion, as well as induced the apoptosis of glioblastoma cells. In the in vivo study, PABPC1 overexpression combined with BDNF-AS overexpression produced the smallest tumor and the longest survival. Moreover, BDNF-AS could elicit retina and anterior neural fold homeobox 2 (RAX2) mRNA decay through STAU1-mediated decay (SMD), and thereby regulated the malignant behaviors glioblastoma cells. Knockdown of RAX2 produced tumor-suppressive function in glioblastoma cells and increased the expression of discs large homolog 5 (DLG5), leading to the activation of the Hippo pathway. In general, this study elucidated that the PABPC1-BDNF-AS-RAX2-DLG5 mechanism may contribute to the anticancer potential of glioma cells and may provide potential therapeutic targets for human glioma.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Knockdown of LncRNA SCAMP1 suppressed malignant biological behaviours of glioma cells via modulating miR-499a-5p/LMX1A/NLRC5 pathway.J Cell Mol Med. 2019 Aug;23(8):5048-5062. doi: 10.1111/jcmm.14362. Epub 2019 Jun 17. J Cell Mol Med. 2019. PMID: 31207033 Free PMC article.
-
LncRNA PRBC induces autophagy to promote breast cancer progression through modulating PABPC1-mediated mRNA stabilization.Oncogene. 2024 Mar;43(14):1019-1032. doi: 10.1038/s41388-024-02971-z. Epub 2024 Feb 16. Oncogene. 2024. PMID: 38366145
-
Lin28A promotes IRF6-regulated aerobic glycolysis in glioma cells by stabilizing SNHG14.Cell Death Dis. 2020 Jun 11;11(6):447. doi: 10.1038/s41419-020-2650-6. Cell Death Dis. 2020. PMID: 32527996 Free PMC article.
-
A concise review on the role of BDNF-AS in human disorders.Biomed Pharmacother. 2021 Oct;142:112051. doi: 10.1016/j.biopha.2021.112051. Epub 2021 Aug 18. Biomed Pharmacother. 2021. PMID: 34426254 Review.
-
Staufen-mediated mRNA decay.Wiley Interdiscip Rev RNA. 2013 Jul-Aug;4(4):423-35. doi: 10.1002/wrna.1168. Epub 2013 May 16. Wiley Interdiscip Rev RNA. 2013. PMID: 23681777 Free PMC article. Review.
Cited by
-
Interaction Between LncRNA and UPF1 in Tumors.Front Genet. 2021 Mar 1;12:624905. doi: 10.3389/fgene.2021.624905. eCollection 2021. Front Genet. 2021. PMID: 33732285 Free PMC article. Review.
-
Predicting potential lncRNA biomarkers for lung cancer and neuroblastoma based on an ensemble of a deep neural network and LightGBM.Front Genet. 2023 Aug 16;14:1238095. doi: 10.3389/fgene.2023.1238095. eCollection 2023. Front Genet. 2023. PMID: 37655066 Free PMC article.
-
Insights into lncRNAs in Alzheimer's disease mechanisms.RNA Biol. 2021 Jul;18(7):1037-1047. doi: 10.1080/15476286.2020.1788848. Epub 2020 Jul 14. RNA Biol. 2021. PMID: 32605500 Free PMC article. Review.
-
The scaffolding protein DLG5 promotes glioblastoma growth by controlling Sonic Hedgehog signaling in tumor stem cells.Neuro Oncol. 2022 Aug 1;24(8):1230-1242. doi: 10.1093/neuonc/noac001. Neuro Oncol. 2022. PMID: 34984467 Free PMC article.
-
ADAR1-Mediated RNA Editing and Its Role in Cancer.Front Cell Dev Biol. 2022 Jul 11;10:956649. doi: 10.3389/fcell.2022.956649. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35898396 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

