Balancing selection via life-history trade-offs maintains an inversion polymorphism in a seaweed fly
- PMID: 32015341
- PMCID: PMC6997199
- DOI: 10.1038/s41467-020-14479-7
Balancing selection via life-history trade-offs maintains an inversion polymorphism in a seaweed fly
Abstract
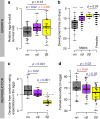
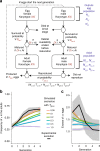
How natural diversity is maintained is an evolutionary puzzle. Genetic variation can be eroded by drift and directional selection but some polymorphisms persist for long time periods, implicating a role for balancing selection. Here, we investigate the maintenance of a chromosomal inversion polymorphism in the seaweed fly Coelopa frigida. Using experimental evolution and quantifying fitness, we show that the inversion underlies a life-history trade-off, whereby each haplotype has opposing effects on larval survival and adult reproduction. Numerical simulations confirm that such antagonistic pleiotropy can maintain polymorphism. Our results also highlight the importance of sex-specific effects, dominance and environmental heterogeneity, whose interaction enhances the maintenance of polymorphism through antagonistic pleiotropy. Overall, our findings directly demonstrate how overdominance and sexual antagonism can emerge from a life-history trade-off, inviting reconsideration of antagonistic pleiotropy as a key part of multi-headed balancing selection processes that enable the persistence of genetic variation.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Brian, Charlesworth & Hugues, K. A. The maintenance of genetic variation in life-history traits. In Evolutionary Genetics from Molecules to Morphology (eds Singh, R. S. & Krimbas, C. B.) 369–392 (Cambridge University Press, Cambridge, 2000).
-
- Prout, Timothy. How well does opposing selection maintain variation? In Evolutionary Genetics From Molecules to Morphology (eds Singh, R. S. & Krimbas, C. B.) 157–181 (Cambridge University Press, Cambridge, 2000).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

