Conformational plasticity of ligand-bound and ternary GPCR complexes studied by 19F NMR of the β1-adrenergic receptor
- PMID: 32015348
- PMCID: PMC6997182
- DOI: 10.1038/s41467-020-14526-3
Conformational plasticity of ligand-bound and ternary GPCR complexes studied by 19F NMR of the β1-adrenergic receptor
Abstract
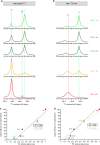
G-protein-coupled receptors (GPCRs) are allosteric signaling proteins that transmit an extracellular stimulus across the cell membrane. Using 19F NMR and site-specific labelling, we investigate the response of the cytoplasmic region of transmembrane helices 6 and 7 of the β1-adrenergic receptor to agonist stimulation and coupling to a Gs-protein-mimetic nanobody. Agonist binding shows the receptor in equilibrium between two inactive states and a pre-active form, increasingly populated with higher ligand efficacy. Nanobody coupling leads to a fully active ternary receptor complex present in amounts correlating directly with agonist efficacy, consistent with partial agonism. While for different agonists the helix 6 environment in the active-state ternary complexes resides in a well-defined conformation, showing little conformational mobility, the environment of the highly conserved NPxxY motif on helix 7 remains dynamic adopting diverse, agonist-specific conformations, implying a further role of this region in receptor function. An inactive nanobody-coupled ternary receptor form is also observed.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

