Comprehensive comparison of Yarrowia lipolytica and Pichia pastoris for production of Candida antarctica lipase B
- PMID: 32015397
- PMCID: PMC6997362
- DOI: 10.1038/s41598-020-58683-3
Comprehensive comparison of Yarrowia lipolytica and Pichia pastoris for production of Candida antarctica lipase B
Abstract
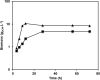
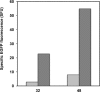
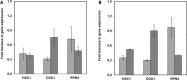
The large-scale production of recombinant proteins (rProt) is becoming increasingly economically important. Among the different hosts used for rProt production, yeasts are gaining popularity. The so-called non-conventional yeasts, such as the methylotrophic Pichia pastoris and the dimorphic Yarrowia lipolytica, are popular choices due to their favorable characteristics and well-established expression systems. Nevertheless, a direct comparison of the two systems for rProt production and secretion was lacking. This study therefore aimed to directly compare Y. lipolytica and P. pastoris for the production and secretion of lipase CalB in bioreactor. Y. lipolytica produced more than double the biomass and more than 5-fold higher extracellular lipase than P. pastoris. Furthermore, maximal CalB production levels were reached by Y. lipolytica in half the cultivation time required for maximal production by P. pastoris. Conversely, P. pastoris was found to express 7-fold higher levels of CalB mRNA. Secreted enhanced green fluorescent protein -in isolation and fused to CalB- and protease inhibitor MG-132 were used in P. pastoris to further investigate the reasons behind such discrepancy. The most likely explanation was ultimately found to be protein degradation by endoplasmic reticulum-associated protein degradation preceding successful secretion. This study highlighted the multifaceted nature of rProt production, prompting a global outlook for selection of rProt production systems.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Çalık P, et al. Recombinant protein production in Pichia pastoris under glyceraldehyde-3-phosphate dehydrogenase promoter: From carbon source metabolism to bioreactor operation parameters. Biochem. Eng. J. 2015;95:20–36. doi: 10.1016/j.bej.2014.12.003. - DOI
-
- Barth, G. & Gaillardin, C. Yarrowia lipolytica. In Nonconventional Yeasts in Biotechnology (ed. Wolf, K.) 313–388, 10.1007/978-3-642-79856-6_10 (Springer Berlin Heidelberg, 1996).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

