Obeticholic acid and INT-767 modulate collagen deposition in a NASH in vitro model
- PMID: 32015483
- PMCID: PMC6997404
- DOI: 10.1038/s41598-020-58562-x
Obeticholic acid and INT-767 modulate collagen deposition in a NASH in vitro model
Abstract
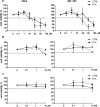
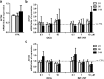
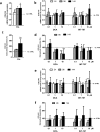
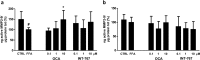
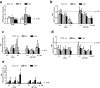
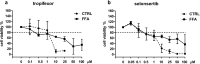
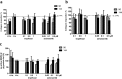
Pharmacological treatments for non-alcoholic steatohepatitis (NASH) are still unsatisfactory. Fibrosis is the most significant predictor of mortality and many anti-fibrotic agents are under evaluation. Herein, we assessed in vitro the effects of the FXR agonist obeticholic acid (OCA) and the dual FXR/TGR5 agonist INT-767 in a well-established co-culture NASH model. Co-cultures of human hepatoma and hepatic stellate (HSCs) cells were exposed to free fatty acids (FFAs) alone or in combination with OCA or INT-767. mRNA expression of HSCs activation markers and FXR engagement were evaluated at 24, 96 and 144 hours. Collagen deposition and metalloproteinase 2 and 9 (MMP2-9) activity were compared to tropifexor and selonsertib. FFAs induced collagen deposition and MMP2-9 activity reduction. Co-treatment with OCA or INT-767 did not affect ACTA2 and COL1A1 expression, but significantly reduced FXR and induced SHP expression, as expected. OCA induced a dose-dependent reduction of collagen and induced MMP2-9 activity. Similarly, INT-767 induced collagen reduction at 96 h and a slight increase in MMP2-9. Tropifexor and Selonsertib were also effective in collagen reduction but showed no modulation of MMP2-9. All tested compounds reduced collagen deposition. OCA exerted a more potent and long-lasting effect, mainly related to modulation of collagen turn-over and MMP2-9 activity.
Conflict of interest statement
This research was funded by Intercept Pharmaceuticals, Inc. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. L.A. is an Intercept Pharmaceutical consultant. B.A., C.T., N.R. have no conflict of interest to declare.
Figures
References
-
- Araújo AR, Rosso N, Bedogni G, Tiribelli C, Bellentani S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. Off. J. Int. Assoc. Study Liver. 2018;38(Suppl 1):47–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

