Regulation and control roles of the basal ganglia in the development of absence epileptiform activities
- PMID: 32015772
- PMCID: PMC6974106
- DOI: 10.1007/s11571-019-09559-4
Regulation and control roles of the basal ganglia in the development of absence epileptiform activities
Abstract
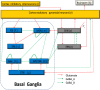
Absence epileptiform activities are traditionally considered to be primarily induced by abnormal interactions between the cortical and thalamic neurons, which form the thalamocortical circuit in the brain. The basal ganglia, as an organizational unit in the brain, has close input and output relationships with the thalamocortical circuit. Although several studies report that the basal ganglia may participate in controlling and regulating absence epileptiform activities, to date, there have been no studies regarding whether the basal ganglia directly cause absence epileptiform activities. In this paper, we built a basal ganglia-corticothalamic network model to determine the role of basal ganglia in this disease. We determined that absence epileptiform activities might be directly induced by abnormal coupling strengths on certain pivotal pathways in the basal ganglia. These epileptiform activities can be well controlled by the coupling strengths of three major pathways that project from the thalamocortical network to the basal ganglia. The results implied that the substantia nigra pars compacta (SNc) can be considered to be the effective treatment target area for inhibiting epileptiform activities, which supports the observations of previous studies. Particularly, as a major contribution of this paper, we determined that the final presentation position of the epileptic slow spike waves is not limited to the cerebral cortex; these waves may additionally appear in the thalamus, striatal medium spiny neurons, striatal fast spiking interneuron, the SNc, subthalamic nucleus, substantia nigra pars reticulata and globus pallidus pars externa. In addition, consistent with several previous studies, the delay in the network was observed to be a critical factor for inducing transitions between different types of absence epileptiform activities. Our new model not only explains the onset and control mechanism but also provides a unified framework to study similar problems in neuron systems.
Keywords: Absence epileptiform activities; Basal ganglia; Onset and control; State transition; Thalamocortical circuit.
© Springer Nature B.V. 2019.
Figures




References
-
- Biraben A, Semah F, Ribeiro MJ, Douaud G, Remy P, Depaulis A. PET evidence for a role of the basal ganglia in patients with ring chromosome 20 epilepsy. Neurology. 2004;63(1):73–77. - PubMed
-
- Bogacz R (2015) Basal Ganglia: beta oscillations. In: Jaeger D, Jung R (eds) Encyclopedia of Computational Neuroscience. Springer, New York, NY, pp 327–330
-
- Breakspear M, Roberts JA, Terry JR, Rodrigues S, Mahant N, Robinson PA. A unifying explanation of primary generalized seizures through nonlinear brain modeling and bifurcation analysis. Cereb Cortex. 2005;16(9):1296–1313. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

