Initial treatment outcome and feasibility of low-dose cabazitaxel against docetaxel- and castration-resistant prostate cancer in a Japanese hospital
- PMID: 32015778
- PMCID: PMC6983453
- DOI: 10.2185/jrm.2019-004
Initial treatment outcome and feasibility of low-dose cabazitaxel against docetaxel- and castration-resistant prostate cancer in a Japanese hospital
Abstract
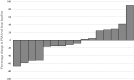
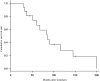
Introduction: Cabazitaxel (CBZ) is used worldwide for castration-resistant prostate cancer after docetaxel treatment. In July 2014 the drug was approved in Japan with the same induction dose used for Caucasian patients. In this study, we examined and compared the results of an initial low-dose CBZ treatment in patients admitted to our hospital. Patients and Methods: Between July 2014 and August 2018, sixteen mCRPC patients were enrolled and underwent a low-dose CBZ treatment at our hospital. We compared the results with those of a Japanese metastatic docetaxel- and castration-resistant prostate cancer Phase I study. Results: The median patient age was 77 years (range, 53-84 years). Of the 16 patients, eight (50%) had a lymph node metastasis and 11 (68.8%) had a distant metastasis, 10 of whom had only a bone metastasis. The median dose of CBZ was 30 mg (range, 20-32 mg) and the median number of CBZ cycles was 2.5 (range, 1-18). The PSA level of 9 (56.3%) patients decreased after CBZ treatment, including 4 (25%) who showed a decrease to <50%. The median time interval in which the PSA level decreased was 2 months (range, 1-18 months). The observed adverse events (AE) were neutropenia (31.3%), febrile neutropenia (6.3%), fatigue (43.8%), nausea (18.8%), diarrhea (12.5%), decreased appetite (25%), dysgeusia (6.3%), white blood cell count decrease (43.8%), platelet count decrease (12.3%), and anemia (75%). However, no patient listed an AE as the reason for discontinuing the treatment. Conclusions: Even at a low dose, CBZ could improve the PSA value in patients with CRPC previously treated with docetaxel. Dose reduction and prophylactic administration of sustained G-CSF were also safe treatment options. Further studies involving an introduction period including a modulation of duration and dose are necessary, especially in Japanese patients.
Keywords: cabazitaxcel; castration resistant prostate cancer; prostate cancer.
©2020 The Japanese Association of Rural Medicine.
Figures
Similar articles
-
The efficacy and toxicity of cabazitaxel for treatment of docetaxel-resistant prostate cancer correlating with the initial doses in Japanese patients.BMC Cancer. 2019 Feb 15;19(1):156. doi: 10.1186/s12885-019-5342-9. BMC Cancer. 2019. PMID: 30770773 Free PMC article.
-
Complete response with early introduction of cabazitaxel in a patient with multiple lung metastases of castration-resistant prostate cancer following the early detection of metastases using liquid biopsy: a case report.BMC Cancer. 2019 Jun 11;19(1):562. doi: 10.1186/s12885-019-5782-2. BMC Cancer. 2019. PMID: 31185946 Free PMC article.
-
Japanese phase I study of cabazitaxel in metastatic castration-resistant prostate cancer.Int J Clin Oncol. 2015 Oct;20(5):1026-34. doi: 10.1007/s10147-015-0820-9. Epub 2015 Mar 26. Int J Clin Oncol. 2015. PMID: 25809824 Clinical Trial.
-
The efficacy of sequential therapy with docetaxel and cabazitaxel for castration-resistant prostate cancer: A retrospective multi-institutional study in Japan.Int J Urol. 2023 Feb;30(2):227-234. doi: 10.1111/iju.15097. Epub 2022 Nov 14. Int J Urol. 2023. PMID: 36375045
-
Risk Factors for Poor Survival in Metastatic Castration-resistant Prostate Cancer Treated With Cabazitaxel in Japan.Anticancer Res. 2019 Oct;39(10):5803-5809. doi: 10.21873/anticanres.13784. Anticancer Res. 2019. PMID: 31570485
References
-
- World Health Organization International Agency for Research on Cancer. Global Cancer Observatory. https://gco.iarc.fr. (Accessed May 14, 2019).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous