Ligand-controlled diastereodivergent, enantio- and regioselective copper-catalyzed hydroxyalkylboration of 1,3-dienes with ketones
- PMID: 32015801
- PMCID: PMC6977547
- DOI: 10.1039/c9sc03531a
Ligand-controlled diastereodivergent, enantio- and regioselective copper-catalyzed hydroxyalkylboration of 1,3-dienes with ketones
Abstract
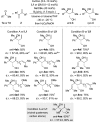
A copper-catalyzed three-component coupling of 1,3-dienes, bis(pinacolato)diboron, and ketones allows for the chemo-, regio-, diastereo- and enantioselective assembly of densely functionalized tertiary homoallylic alcohols. The relative configuration of the vicinal stereocenters is controlled by the chiral ligand employed. Subsequent transformations illustrate the versatility of these valuable chiral building blocks.
This journal is © The Royal Society of Chemistry 2019.
Figures



References
-
-
For reviews of related asymmetric allylation of carbonyl compounds, see:
- Liu Y.-L., Lin X.-T. Adv. Synth. Catal. 2019;361:876–918.
- Hatano M., Ishihara K. Synthesis. 2008:1647–1675.
- Denmark S. E., Fu J. Chem. Rev. 2003;103:2763–2793. - PubMed
-
-
- Yus M., González-Gómez J. C., Foubelo F. Chem. Rev. 2013;113:5595–5698. - PubMed
-
-
With allylboron reagents:
- Wada R., Oisaki K., Kanai M., Shibasaki M. J. Am. Chem. Soc. 2004;126:8910–8911. - PubMed
- Lou S., Moquist P. N., Schaus S. E. J. Am. Chem. Soc. 2006;128:12660–12661. - PubMed
- Schneider U., Ueno M., Kobayashi S. J. Am. Chem. Soc. 2008;130:13824–13825. - PubMed
- Shi S.-L., Xu L.-W., Oisaki K., Kanai M., Shibasaki M. J. Am. Chem. Soc. 2010;132:6638–6639. - PubMed
- Barnett D. S., Moquist P. N., Schaus S. E. Angew. Chem., Int. Ed. 2009;48:8679–8682. - PMC - PubMed
- Alam R., Vollgraff T., Eriksson L., Szabó K. J. J. Am. Chem. Soc. 2015;137:11262–11265. - PubMed
- Robbins D. W., Lee K. A., Silverio D. L., Volkov A., Torker S., Hoveyda A. H. Angew. Chem., Int. Ed. 2016;55:9610–9614. - PMC - PubMed
- Lee K. A., Silverio D. L., Torker S., Robbins D. W., Haeffner F., van der Mei F. W., Hoveyda A. H. Nat. Chem. 2016;8:768–777. - PMC - PubMed
- van der Mei F. W., Qin C., Morrison R. J., Hoveyda A. H. J. Am. Chem. Soc. 2017;139:9053–9065. - PMC - PubMed
-
-
-
With allyltin reagents:
- Casolari S., D'Addario D., Tagliavini E. Org. Lett. 1999;1:1061–1063.
- Waltz K. M., Gavenonis J., Walsh P. J. Angew. Chem., Int. Ed. 2002;41:3697–3600. - PubMed
- Cunningham A., Woodward S. Synthesis. 2002:43–44.
- Kim J. G., Waltz K. M., Garcia I. F., Kwiatkowski D., Walsh P. J. J. Am. Chem. Soc. 2004;126:12580–12585. - PubMed
- Teo Y.-C., Goh J.-D., Loh T.-P. Org. Lett. 2005;7:2743–2745. - PubMed
- Kim J. G., Camp E. H., Walsh P. J. Org. Lett. 2006;8:4413–4416. - PMC - PubMed
- Zhang X., Chen D., Liu X., Feng X. J. Org. Chem. 2007;72:5227–5233. - PubMed
-
LinkOut - more resources
Full Text Sources
Miscellaneous

