Ureidopeptide GLP-1 analogues with prolonged activity in vivo via signal bias and altered receptor trafficking
- PMID: 32015811
- PMCID: PMC6977461
- DOI: 10.1039/c9sc02079a
Ureidopeptide GLP-1 analogues with prolonged activity in vivo via signal bias and altered receptor trafficking
Abstract
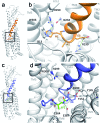
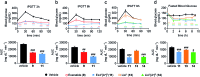
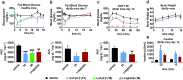
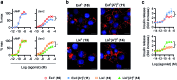
The high demand of the pharmaceutical industry for new modalities to address the diversification of biological targets with large surfaces of interaction led us to investigate the replacement of α-amino acid residues with ureido units at selected positions in peptides to improve potency and generate effective incretin mimics. Based on molecular dynamics simulations, N-terminally modified GLP-1 analogues with a ureido residue replacement at position 2 were synthesized and showed preservation of agonist activity while exhibiting a substantial increase in stability. This enabling platform was applied to exenatide and lixisenatide analogues to generate two new ureidopeptides with antidiabetic properties and longer duration of action. Further analyses demonstrated that the improvement was due mainly to differences in signal bias and trafficking of the GLP-1 receptor. This study demonstrates the efficacy of single α-amino acid substitution with ureido residues to design long lasting peptides.
This journal is © The Royal Society of Chemistry 2019.
Figures


References
-
- Erak M., Bellmann-Sickert K., Els-Heindl S., Beck-Sickinger A. G. Bioorg. Med. Chem. 2018;26:2759–2765. - PubMed
-
- Valeur E., Guéret S. M., Adihou H., Gopalakrishnan R., Lemurell M., Waldmann H., Grossmann T. N., Plowright A. T. Angew. Chem., Int. Ed. 2017;56:10294–10323. - PubMed
-
- Chingle R., Proulx C., Lubell W. D. Acc. Chem. Res. 2017;50:1541–1556. - PubMed
-
- Zhang Y., Malamakal R. M., Chenoweth D. M. J. Am. Chem. Soc. 2015;137:12422–12425. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

