IRMOF-74(n)-Mg: a novel catalyst series for hydrogen activation and hydrogenolysis of C-O bonds
- PMID: 32015812
- PMCID: PMC6977460
- DOI: 10.1039/c9sc01018a
IRMOF-74(n)-Mg: a novel catalyst series for hydrogen activation and hydrogenolysis of C-O bonds
Abstract
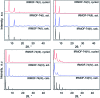
Metal-Organic Frameworks (MOFs) that catalyze hydrogenolysis reactions are rare and there is little understanding of how the MOF, hydrogen, and substrate molecules interact. In this regard, the isoreticular IRMOF-74 series, two of which are known catalysts for hydrogenolysis of aromatic C-O bonds, provides an unusual opportunity for systematic probing of these reactions. The diameter of the 1D open channels can be varied within a common topology owing to the common secondary building unit (SBU) and controllable length of the hydroxy-carboxylate struts. We show that the first four members of the IRMOF-74(Mg) series are inherently catalytic for aromatic C-O bond hydrogenolysis and that the conversion varies non-monotonically with pore size. These catalysts are recyclable and reusable, retaining their crystallinity and framework structure after the hydrogenolysis reaction. The hydrogenolysis conversion of phenylethylphenyl ether (PPE), benzylphenyl ether (BPE), and diphenyl ether (DPE) varies as PPE > BPE > DPE, consistent with the strength of the C-O bond. Counterintuitively, however, the conversion also follows the trend IRMOF-74(III) > IRMOF-74(IV) > IRMOF-74(II) > IRMOF-74(I), with little variation in the corresponding selectivity. DFT calculations suggest the unexpected behavior is due to much stronger ether and phenol binding to the Mg(ii) open metal sites (OMS) of IRMOF-74(III), resulting from a structural distortion that moves the Mg2+ ions toward the interior of the pore. Solid-state 25Mg NMR data indicate that both H2 and ether molecules interact with the Mg(ii) OMS and hydrogen-deuterium exchange reactions show that these MOFs activate dihydrogen bonds. The results suggest that both confinement and the presence of reactive metals are essential for achieving the high catalytic activity, but that subtle variations in pore structure can significantly affect the catalysis. Moreover, they challenge the notion that simply increasing MOF pore size within a constant topology will lead to higher conversions.
This journal is © The Royal Society of Chemistry 2019.
Figures




Similar articles
-
Revisiting isoreticular MOFs of alkaline earth metals: a comprehensive study on phase stability, electronic structure, chemical bonding, and optical properties of A-IRMOF-1 (A = Be, Mg, Ca, Sr, Ba).Phys Chem Chem Phys. 2011 Jun 7;13(21):10191-203. doi: 10.1039/c0cp02944k. Epub 2011 Apr 19. Phys Chem Chem Phys. 2011. PMID: 21503357
-
Mechanism of Ni N-heterocyclic carbene catalyst for C-O bond hydrogenolysis of diphenyl ether: a density functional study.Dalton Trans. 2014 Dec 28;43(48):18123-33. doi: 10.1039/c4dt02374a. Dalton Trans. 2014. PMID: 25355042
-
Bimetallic nickel‑cobalt sulfide derived from lignin-MOFs hybrid as a high-efficiency heterogeneous catalyst for hydrogenolysis of lignin dimers.Int J Biol Macromol. 2025 Jan;287:138658. doi: 10.1016/j.ijbiomac.2024.138658. Epub 2024 Dec 10. Int J Biol Macromol. 2025. PMID: 39667449
-
Isoreticular Metal-Organic Framework-3 (IRMOF-3): From Experimental Preparation, Functionalized Modification to Practical Applications.Polymers (Basel). 2024 Jul 26;16(15):2134. doi: 10.3390/polym16152134. Polymers (Basel). 2024. PMID: 39125160 Free PMC article. Review.
-
Metal-Organic Framework (MOF)-Based Materials as Heterogeneous Catalysts for C-H Bond Activation.Chemistry. 2019 Feb 26;25(12):2935-2948. doi: 10.1002/chem.201804149. Epub 2018 Dec 13. Chemistry. 2019. PMID: 30264533 Review.
Cited by
-
High-Throughput Experimentation, Theoretical Modeling, and Human Intuition: Lessons Learned in Metal-Organic-Framework-Supported Catalyst Design.ACS Cent Sci. 2023 Jan 26;9(2):266-276. doi: 10.1021/acscentsci.2c01422. eCollection 2023 Feb 22. ACS Cent Sci. 2023. PMID: 36844483 Free PMC article.
-
Solid-state NMR spectroscopy: an advancing tool to analyse the structure and properties of metal-organic frameworks.Chem Sci. 2020 Apr 6;11(17):4297-4304. doi: 10.1039/d0sc00735h. Chem Sci. 2020. PMID: 34122887 Free PMC article. Review.
-
Challenges to developing materials for the transport and storage of hydrogen.Nat Chem. 2022 Nov;14(11):1214-1223. doi: 10.1038/s41557-022-01056-2. Epub 2022 Oct 27. Nat Chem. 2022. PMID: 36302871 Review.
-
Visible-light photoredox-catalyzed C-O bond cleavage of diaryl ethers by acridinium photocatalysts at room temperature.Nat Commun. 2020 Nov 30;11(1):6126. doi: 10.1038/s41467-020-19944-x. Nat Commun. 2020. PMID: 33257656 Free PMC article.
-
MOFs-Modified Electrochemical Sensors and the Application in the Detection of Opioids.Biosensors (Basel). 2023 Feb 16;13(2):284. doi: 10.3390/bios13020284. Biosensors (Basel). 2023. PMID: 36832051 Free PMC article. Review.
References
-
- Thring R. W., Breau J. Fuel. 1996;75:795–800.
-
- Higman C., Tam S. Chem. Rev. 2014;114:1673–1708. - PubMed
-
- Andrews J., Shabani B. Wiley Interdiscip. Rev.: Energy Environ. 2014;3:474–489.
-
- Züttel A., Remhof A., Borgschulte A., Friedrichs O. Philos. Trans. R. Soc., A. 2010;368:3329–3342. - PubMed
-
- Ruppert A. M., Weinberg K., Palkovits R. Angew. Chem., Int. Ed. 2012;51:2564–2601. - PubMed
LinkOut - more resources
Full Text Sources

