Food quality effects on instar-specific life histories of a holometabolous insect
- PMID: 32015831
- PMCID: PMC6988550
- DOI: 10.1002/ece3.5790
Food quality effects on instar-specific life histories of a holometabolous insect
Abstract
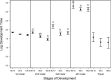
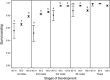
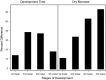
It is a long-standing challenge to understand how changes in food resources impact consumer life history traits and, in turn, impact how organisms interact with their environment. To characterize food quality effects on life history, most studies follow organisms throughout their life cycle and quantify major life events, such as age at maturity or fecundity. From these studies, we know that food quality generally impacts body size, juvenile development, and life span. Importantly, throughout juvenile development, many organisms develop through several stages of growth that can have different interactions with their environment. For example, some parasitoids typically attack larger instars, whereas larval insect predators typically attack smaller instars. Interestingly, most studies lump all juvenile stages together, which ignores these ecological changes over juvenile development.We combine a cross-sectional experimental approach with a stage-structured population model to estimate instar-specific vital rates in the bean weevil, Callosobruchus maculatus across a food quality gradient. We characterize food quality effects on the bean weevil's life history traits throughout its juvenile ontogeny to test how food quality impacts instar-specific vital rates.Vital rates differed across food quality treatments within each instar; however, their effect differed with instar. Weevils consuming low-quality food spent 38%, 37%, and 18% more time, and were 34%, 53%, and 63% smaller than weevils consuming high-quality food in the second, third, and fourth instars, respectively. Overall, our results show that consuming poor food quality means slower growth, but that food quality effects on vital rates, growth and development are not equal across instars. Differences in life history traits over juvenile ontogeny in response to food quality may impact how organisms interact with their environment, including how susceptible they are to predation, parasitism, and their competitive ability.
Keywords: cowpea weevil; juvenile ontogeny; phytophagous consumer; stage‐structured population model.
© 2019 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures




References
-
- Barrigossi, J. A. F. , Zimmermann, F. J. P. , & Lima, P. D. C. (2002). Consumption rates and performance of Erinnyis ello L. on four cassava varieties. Neotropical Entomology, 31, 10.1590/S1519-566X2002000300012 - DOI
-
- Bauce, E. , Bidon, Y. , & Berthiaume, R. (2002). Effects of food nutritive quality and Bacillus thuringiensis on feeding behavior, food utilization and larval growth of spruce budworm Choristoneura fumiferana (Clem.) when exposed as fourth‐ and sixth‐instar larvae. Agricultural and Forest Entomology, 4, 57–70.
-
- Benavides, A. G. , Cancino, J. M. , & Ojeda, F. P. (1994). Ontogenetic changes in gut dimensions and macroalgal digestibility in the marine herbivorous fish, Aplodactylus punctatus. Functional Ecology, 8, 46–51. 10.2307/2390110 - DOI
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

