6'-Methoxy Raloxifene-analog enhances mouse bone properties with reduced estrogen receptor binding
- PMID: 32016137
- PMCID: PMC6992940
- DOI: 10.1016/j.bonr.2020.100246
6'-Methoxy Raloxifene-analog enhances mouse bone properties with reduced estrogen receptor binding
Abstract
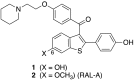
Raloxifene (RAL) is an FDA-approved drug used to treat osteoporosis in postmenopausal women. RAL suppresses bone loss primarily through its role as a selective estrogen receptor modulator (SERM). This hormonal estrogen therapy promotes unintended side effects, such as hot flashes and increased thrombosis risk, and prevents the drug from being used in some patient populations at-risk for fracture, including children with bone disorders. It has recently been demonstrated that RAL can have significant positive effects on overall bone mechanical properties by binding to collagen and increasing bone tissue hydration in a cell-independent manner. A Raloxifene-Analog (RAL-A) was synthesized by replacing the 6-hydroxyl substituent with 6-methoxy in effort to reduce the compound's binding affinity for estrogen receptors (ER) while maintaining its collagen-binding ability. It was hypothesized that RAL-A would improve the mechanical integrity of bone in a manner similar to RAL, but with reduced estrogen receptor binding. Molecular assessment showed that while RAL-A did reduce ER binding, downstream ER signaling was not completely abolished. In-vitro, RAL-A performed similarly to RAL and had an identical concentration threshold on osteocyte cell proliferation, differentiation, and function. To assess treatment effect in-vivo, wildtype (WT) and heterozygous (OIM+/-) female mice from the Osteogenesis Imperfecta (OI) murine model were treated with either RAL or RAL-A from 8 weeks to 16 weeks of age. There was an untreated control group for each genotype as well. Bone microarchitecture was assessed using microCT, and mechanical behavior was assessed using 3-point bending. Results indicate that both compounds produced analogous gains in tibial trabecular and cortical microarchitecture. While WT mechanical properties were not drastically altered with either treatment, OIM+/- mechanical properties were significantly enhanced, most notably, in post-yield properties including bone toughness. This proof-of-concept study shows promising results and warrants the exploration of additional analog iterations to further reduce ER binding and improve fracture resistance.
Keywords: Bone mechanics; Bone quality; Osteogenesis Imperfecta; SERM.
© 2020 The Authors.
Conflict of interest statement
There are no known conflicts of interest associated with this publication, and there has been no financial support for this work that could have influenced its outcome.
Figures




References
-
- Ablenas F.J., George B.E., Maleki M., Jain R., Hopkinson A.C., Lee-Ruff E. Destabilized carbocations. Nuclear magnetic resonance detection and reactivities of aryl α-thioformamidyl cations. Can. J. Chem. 2011;65:1800–1803.
-
- Acevedo C. Alendronate treatment alters bone tissues at multiple structural levels in healthy canine cortical bone. Bone. 2015;81:352–363. (Dec) - PubMed
-
- Allen M.R., Burr D.B. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don’t know. Bone. 2011;49(1):56–65. (Jul) - PubMed
-
- Allen M.R., Iwata K., Phipps R., Burr D.B. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39(4):872–879. (Oct) - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

