Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions
- PMID: 32016537
- PMCID: PMC7224136
- DOI: 10.1007/s00134-020-05942-6
Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions
Abstract
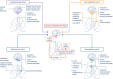
Neural respiratory drive, i.e., the activity of respiratory centres controlling breathing, is an overlooked physiologic variable which affects the pathophysiology and the clinical outcome of acute respiratory distress syndrome (ARDS). Spontaneous breathing may offer multiple physiologic benefits in these patients, including decreased need for sedation, preserved diaphragm activity and improved cardiovascular function. However, excessive effort to breathe due to high respiratory drive may lead to patient self-inflicted lung injury (P-SILI), even in the absence of mechanical ventilation. In the present review, we focus on the physiological and clinical implications of control of respiratory drive in ARDS patients. We summarize the main determinants of neural respiratory drive and the mechanisms involved in its potentiation, in health and ARDS. We also describe potential and pitfalls of the available bedside methods for drive assessment and explore classical and more "futuristic" interventions to control drive in ARDS patients.
Conflict of interest statement
ES and JRB do not have any conflict of interests to disclose. TM reports personal fees from Drager, Fisher and Paykel and Mindray, outside the submitted work. AP reports personal fees from Maquet, Novalung/Xenios, Baxter and Boehringer Ingelheim, outside the submitted work. DB reports grants from ALung technologies, personal fees from Baxter, personal fees from BREETHE, personal fees from Xenios, other from Hemovent, outside the submitted work.
Figures


Comment in
-
Dissociation between the brain target and respiratory capacity in critically ill patients. Authors' reply.Intensive Care Med. 2020 May;46(5):1079-1080. doi: 10.1007/s00134-020-05984-w. Epub 2020 Mar 3. Intensive Care Med. 2020. PMID: 32125454 No abstract available.
-
Dissociation between the brain target and respiratory capacity in critically ill patients.Intensive Care Med. 2020 May;46(5):1077-1078. doi: 10.1007/s00134-020-05983-x. Epub 2020 Mar 5. Intensive Care Med. 2020. PMID: 32140735 No abstract available.
References
-
- Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. - PubMed
-
- Smith CA, Rodman JR, Chenuel BJ, Henderson KS, Dempsey JA (2006) Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol (1985) 100:13–19 - PubMed
-
- Mohan R, Duffin J. The effect of hypoxia on the ventilatory response to carbon dioxide in man. Respir Physiol. 1997;108:101–115. - PubMed
-
- Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, Martino P. Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol. 2000;119:199–208. - PubMed

