Slow ring flips in aromatic cluster of GB1 studied by aromatic 13C relaxation dispersion methods
- PMID: 32016706
- PMCID: PMC7080667
- DOI: 10.1007/s10858-020-00303-3
Slow ring flips in aromatic cluster of GB1 studied by aromatic 13C relaxation dispersion methods
Abstract
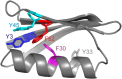
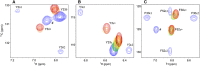
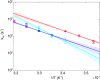
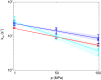
Ring flips of phenylalanine and tyrosine are a hallmark of protein dynamics. They report on transient breathing motions of proteins. In addition, flip rates also depend on stabilizing interactions in the ground state, like aromatic stacking or cation-π interaction. So far, experimental studies of ring flips have almost exclusively been performed on aromatic rings without stabilizing interactions. Here we investigate ring flip dynamics of Phe and Tyr in the aromatic cluster in GB1. We found that all four residues of the cluster, Y3, F30, Y45 and F52, display slow ring flips. Interestingly, F52, the central residue of the cluster, which makes aromatic contacts with all three others, is flipping significantly faster, while the other rings are flipping with the same rates within margin of error. Determined activation enthalpies and activation volumes of these processes are in the same range of other reported ring flips of single aromatic rings. There is no correlation of the number of aromatic stacking interactions to the activation enthalpy, and no correlation of the ring's extent of burying to the activation volume. Because of these findings, we speculate that F52 is undergoing concerted ring flips with each of the other rings.
Keywords: Aromatic interaction; NMR spectroscopy; Protein breathing; Protein dynamics; Protein stability.
Figures


Similar articles
-
Ring flips revisited: (13)C relaxation dispersion measurements of aromatic side chain dynamics and activation barriers in basic pancreatic trypsin inhibitor.Biochemistry. 2014 Jul 22;53(28):4519-25. doi: 10.1021/bi500462k. Epub 2014 Jul 11. Biochemistry. 2014. PMID: 24983918
-
Slow aromatic ring flips detected despite near-degenerate NMR frequencies of the exchanging nuclei.J Phys Chem B. 2013 Aug 8;117(31):9241-7. doi: 10.1021/jp4058065. Epub 2013 Jul 29. J Phys Chem B. 2013. PMID: 23859599
-
Aromatic ring-flipping in supercooled water: implications for NMR-based structural biology of proteins.J Am Chem Soc. 2001 Jan 24;123(3):388-97. doi: 10.1021/ja003220l. J Am Chem Soc. 2001. PMID: 11456540
-
Changes in the Aqueous Solvent do not Impact the Internal Ring-Flip Dynamic of Fully Buried F52 in Protein GB1.Chembiochem. 2025 Jul 11;26(13):e202500183. doi: 10.1002/cbic.202500183. Epub 2025 Jun 4. Chembiochem. 2025. PMID: 40375829 Free PMC article.
-
NMR Studies of Aromatic Ring Flips to Probe Conformational Fluctuations in Proteins.J Phys Chem B. 2023 Jan 26;127(3):591-599. doi: 10.1021/acs.jpcb.2c07258. Epub 2023 Jan 14. J Phys Chem B. 2023. PMID: 36640108 Free PMC article. Review.
Cited by
-
Direct observation of chemo-mechanical coupling in DnaK by single-molecule force experiments.Biophys J. 2022 Dec 6;121(23):4729-4739. doi: 10.1016/j.bpj.2022.09.042. Epub 2022 Oct 3. Biophys J. 2022. PMID: 36196054 Free PMC article.
-
Characterizing Fast Conformational Exchange of Aromatic Rings Using Residual Dipolar Couplings: Distinguishing Jumplike Flips from Other Exchange Mechanisms.J Phys Chem B. 2022 Oct 13;126(40):7950-7956. doi: 10.1021/acs.jpcb.2c05097. Epub 2022 Sep 30. J Phys Chem B. 2022. PMID: 36180044 Free PMC article.
-
Pepsin-like aspartic proteases (PAPs) as model systems for combining biomolecular simulation with biophysical experiments.RSC Adv. 2021 Mar 17;11(18):11026-11047. doi: 10.1039/d0ra10359d. eCollection 2021 Mar 10. RSC Adv. 2021. PMID: 35423571 Free PMC article. Review.
-
Visualizing protein breathing motions associated with aromatic ring flipping.Nature. 2022 Feb;602(7898):695-700. doi: 10.1038/s41586-022-04417-6. Epub 2022 Feb 16. Nature. 2022. PMID: 35173330 Free PMC article.
-
1H R1ρ relaxation dispersion experiments in aromatic side chains.J Biomol NMR. 2021 Dec;75(10-12):383-392. doi: 10.1007/s10858-021-00382-w. Epub 2021 Sep 12. J Biomol NMR. 2021. PMID: 34510298 Free PMC article.
References
-
- Burley SK, Petsko GA. Electrostatic interactions in aromatic oligopeptides contribute to protein stability. Trends Biotechnol. 1989;7:354–359. doi: 10.1016/0167-7799(89)90036-X. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

