Establishment and evolution of heterochromatin
- PMID: 32017156
- PMCID: PMC7586837
- DOI: 10.1111/nyas.14303
Establishment and evolution of heterochromatin
Abstract
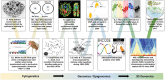
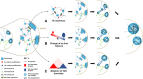
The eukaryotic genome is packaged into transcriptionally active euchromatin and silent heterochromatin, with most studies focused on the former encompassing the majority of protein-coding genes. The recent development of various sequencing techniques has refined this classic dichromatic partition and has better illuminated the composition, establishment, and evolution of this genomic and epigenomic "dark matter" in the context of topologically associated domains and phase-separated droplets. Heterochromatin includes genomic regions that can be densely stained by chemical dyes, which have been shown to be enriched for repetitive elements and epigenetic marks, including H3K9me2/3 and H3K27me3. Heterochromatin is usually replicated late, concentrated at the nuclear periphery or around nucleoli, and usually lacks highly expressed genes; and now it is considered to be as neither genetically inert nor developmentally static. Heterochromatin guards genome integrity against transposon activities and exerts important regulatory functions by targeting beyond its contained genes. Both its nucleotide sequences and regulatory proteins exhibit rapid coevolution between species. In addition, there are dynamic transitions between euchromatin and heterochromatin during developmental and evolutionary processes. We summarize here the ever-changing characteristics of heterochromatin and propose models and principles for the evolutionary transitions of heterochromatin that have been mainly learned from studies of Drosophila and yeast. Finally, we highlight the role of sex chromosomes in studying heterochromatin evolution.
Keywords: chromatin conformation; heterochromatin; histone modifications; sex chromosomes.
© 2020 The Authors. Annals of the New York Academy of Sciences published by Wiley Periodicals Inc. on behalf of New York Academy of Sciences.
Figures

Similar articles
-
Molecular landscape of modified histones in Drosophila heterochromatic genes and euchromatin-heterochromatin transition zones.PLoS Genet. 2008 Jan;4(1):e16. doi: 10.1371/journal.pgen.0040016. Epub 2007 Dec 13. PLoS Genet. 2008. PMID: 18208336 Free PMC article.
-
Adaptation of gene loci to heterochromatin in the course of Drosophila evolution is associated with insulator proteins.Sci Rep. 2020 Jul 17;10(1):11893. doi: 10.1038/s41598-020-68879-2. Sci Rep. 2020. PMID: 32681087 Free PMC article.
-
Enrichment of HP1a on Drosophila chromosome 4 genes creates an alternate chromatin structure critical for regulation in this heterochromatic domain.PLoS Genet. 2012 Sep;8(9):e1002954. doi: 10.1371/journal.pgen.1002954. Epub 2012 Sep 20. PLoS Genet. 2012. PMID: 23028361 Free PMC article.
-
Histone modification and the control of heterochromatic gene silencing in Drosophila.Chromosome Res. 2006;14(4):377-92. doi: 10.1007/s10577-006-1066-1. Chromosome Res. 2006. PMID: 16821134 Review.
-
The paradox of functional heterochromatin.Bioessays. 2005 Jan;27(1):29-41. doi: 10.1002/bies.20158. Bioessays. 2005. PMID: 15612038 Review.
Cited by
-
Nuclear Organization during Hepatogenesis in Zebrafish Requires Uhrf1.Genes (Basel). 2021 Jul 16;12(7):1081. doi: 10.3390/genes12071081. Genes (Basel). 2021. PMID: 34356097 Free PMC article.
-
BET Epigenetic Reader Proteins in Cardiovascular Transcriptional Programs.Circ Res. 2020 Apr 24;126(9):1190-1208. doi: 10.1161/CIRCRESAHA.120.315929. Epub 2020 Apr 23. Circ Res. 2020. PMID: 32324495 Free PMC article. Review.
-
Evaluation of chromatin mesoscale organization.APL Bioeng. 2022 Jan 12;6(1):010902. doi: 10.1063/5.0069286. eCollection 2022 Mar. APL Bioeng. 2022. PMID: 35071965 Free PMC article.
-
Tandem Repeat Diversity in Two Closely Related Hamster Species-The Chinese Hamster (Cricetulus griseus) and Striped Hamster (Cricetulus barabensis).Biomedicines. 2022 Apr 18;10(4):925. doi: 10.3390/biomedicines10040925. Biomedicines. 2022. PMID: 35453675 Free PMC article.
-
Population Epigenetics: The Extent of DNA Methylation Variation in Wild Animal Populations.Epigenomes. 2022 Sep 28;6(4):31. doi: 10.3390/epigenomes6040031. Epigenomes. 2022. PMID: 36278677 Free PMC article. Review.
References
-
- Heitz, E. 1928. Das Heterochromatin der Moose. Jahrb. Wiss. Bot. 69: 762–818.
-
- Mcclung, C.E. 1902. The accessory chromosome—sex determinant? Biol. Bull. 3: 43–84.
-
- Sutton, W.S. 1902. On the morphology of the chromosome group in Brachystola magna . Biol. Bull. 4: 24–39.
-
- Gatti, M. , Pimpinelli S. & Santini G.. 1976. Characterization of Drosophila heterochromatin. Chromosoma 57: 351–375. - PubMed
-
- Pimpinelli, S. , Gatti M. & De Marco A.. 1975. Evidence for heterogeneity in heterochromatin of Drosophila melanogaster . Nature 256: 335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

