Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients With Cirrhosis
- PMID: 32017165
- PMCID: PMC7398837
- DOI: 10.1002/hep.31159
Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients With Cirrhosis
Abstract
Background and aims: There are limited data on hepatocellular carcinoma (HCC) growth patterns, particularly in Western cohorts, despite implications for surveillance, prognosis, and treatment. Our study's aim was to quantify tumor doubling time (TDT) and identify correlates associated with indolent and rapid growth.
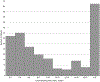
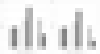
Approach and results: We performed a retrospective multicenter cohort study of patients with cirrhosis diagnosed with HCC from 2008 to 2017 at six US and European health systems with two or more contrast-enhanced imaging studies performed ≥ 30 days apart prior to HCC treatment. Radiologists independently measured tumors in three dimensions to calculate TDT and specific growth rate (SGR). We used multivariable ordinal logistic regression to identify factors associated with indolent (TDT > 365 days) and rapid (TDT < 90 days) tumor growth. In the primary cohort (n = 242 patients from four centers), median TDT was 229 days (interquartile range [IQR], 89-627) and median SGR was 0.3% per day (IQR, 0.1%-0.8%). Over one-third (38%) of HCCs had indolent growth, 36.8% intermediate growth, and 25.2% rapid growth. In multivariable analysis, indolent growth was associated with larger tumor diameter (odds ratio [OR], 1.15, 95% confidence interval [CI], 1.03-1.30) and alpha-fetoprotein < 20 ng/mL (OR, 1.90; 95% CI, 1.12-3.21). Indolent growth was more common in nonviral than viral cirrhosis (50.9% versus 32.1%), particularly in patients with T1 HCC (OR, 3.41; 95% CI, 1.08-10.80). Median TDT (169 days; IQR 74-408 days) and SGR (0.4% per day) were similar in an independent cohort (n = 176 patients from two centers).
Conclusions: In a large Western cohort of patients with HCC, we found heterogeneous tumor growth patterns, with one-fourth exhibiting rapid growth and over one-third having indolent growth. Better understanding different tumor growth patterns may facilitate a precision approach to prognostication and treatment.
© 2020 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures

Comment in
-
Letter to the Editor: Should Growth Pattern of Hepatocellular Carcinoma Be Constant and Homogenous?Hepatology. 2021 Jun;73(6):2617-2618. doi: 10.1002/hep.31640. Hepatology. 2021. PMID: 33205459 No abstract available.
-
REPLY.Hepatology. 2021 Jun;73(6):2618-2619. doi: 10.1002/hep.31638. Epub 2021 May 17. Hepatology. 2021. PMID: 33205463 Free PMC article. No abstract available.
References
-
- Tamura S, Kato T, Berho M, et al. Impact of Histological Grade of Hepatocellular Carcinoma on the Outcome of Liver Transplantation. JAMA Surgery 2001;136:25–30. - PubMed
-
- Lim K-C, Chow PK-H, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Annals of surgery 2011;254:108–113. - PubMed
-
- Sandow TA, Arndt SE, Albar AA, et al. Assessment of Response to Transcatheter Arterial Chemoembolization with Doxorubicin-eluting Microspheres: Tumor Biology and Hepatocellular Carcinoma Recurrence in a 5-year Transplant Cohort. Radiology 2018;286:1072–1083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

