Legal regulations, ethical guidelines and recent policies to increase transparency of clinical trials
- PMID: 32017178
- PMCID: PMC7098869
- DOI: 10.1111/bcp.14223
Legal regulations, ethical guidelines and recent policies to increase transparency of clinical trials
Abstract
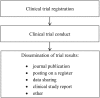
Timely and accurate dissemination of outcomes is essential to accomplish main benefits of scientific research including clinical trials. Clinical trial results can be disseminated in two main ways: by publication in a peer-reviewed journal and by posting on a publicly available clinical trial register. The credibility of the literature on clinical trials is significantly diminished because a high percentage of trials is not published. While current legal regulations both in the European Union (EU) and the USA impose a duty to submit summary results of clinical trials to a respective register (EU Clinical Trial Register and ClinicalTrials.gov, respectively), the compliance with this requirement has been generally inadequate. Trial outcomes can be also made accessible by data sharing. However, in spite of the wide promotion of this idea, the access of investigators to participant-level datasets remains limited. The main objective of this review is to discuss current legal regulations, international standards, ethical guidelines and recent policies pertaining to dissemination of clinical trial results.
Keywords: ClinicalTrials.gov; EU Clinical Trial Register; clinical trial; clinical trial register; data sharing.
© 2020 The British Pharmacological Society.
Conflict of interest statement
There are no competing interests to declare.
Figures
References
-
- CONSORT . CONSORT Statement. http://www.consort-statement.org/ Accessed October 1, 2019
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

