Dynamics of transmissible gastroenteritis virus internalization unraveled by single-virus tracking in live cells
- PMID: 32017270
- PMCID: PMC7163995
- DOI: 10.1096/fj.201902455R
Dynamics of transmissible gastroenteritis virus internalization unraveled by single-virus tracking in live cells
Abstract
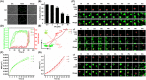
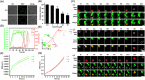
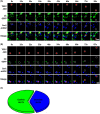
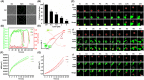
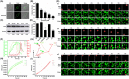
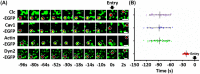
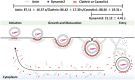
Transmissible gastroenteritis virus (TGEV) is a swine enteropathogenic coronavirus that causes significant economic losses in swine industry. Current studies on TGEV internalization mainly focus on viral receptors, but the internalization mechanism is still unclear. In this study, we used single-virus tracking to obtain the detailed insights into the dynamic events of the TGEV internalization and depict the whole sequential process. We observed that TGEVs could be internalized through clathrin- and caveolae-mediated endocytosis, and the internalization of TGEVs was almost completed within ~2 minutes after TGEVs attached to the cell membrane. Furthermore, the interactions of TGEVs with actin and dynamin 2 in real time during the TGEV internalization were visualized. To our knowledge, this is the first report that single-virus tracking technique is used to visualize the entire dynamic process of the TGEV internalization: before the TGEV internalization, with the assistance of actin, clathrin, and caveolin 1 would gather around the virus to form the vesicle containing the TGEV, and after ~60 seconds, dynamin 2 would be recruited to promote membrane fission. These results demonstrate that TGEVs enter ST cells via clathrin- and caveolae-mediated endocytic, actin-dependent, and dynamin 2-dependent pathways.
Keywords: TGEV; actin; caveolae; clathrin; dynamin 2.
© 2020 Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declared that they have no conflicts of interest to this work.
Figures

Similar articles
-
Epidermal growth factor receptor is a co-factor for transmissible gastroenteritis virus entry.Virology. 2018 Aug;521:33-43. doi: 10.1016/j.virol.2018.05.009. Epub 2018 Jun 4. Virology. 2018. PMID: 29879540 Free PMC article.
-
The coronavirus transmissible gastroenteritis virus causes infection after receptor-mediated endocytosis and acid-dependent fusion with an intracellular compartment.J Virol. 1998 Jan;72(1):527-34. doi: 10.1128/JVI.72.1.527-534.1998. J Virol. 1998. PMID: 9420255 Free PMC article.
-
Dynamic Dissection of the Endocytosis of Porcine Epidemic Diarrhea Coronavirus Cooperatively Mediated by Clathrin and Caveolae as Visualized by Single-Virus Tracking.mBio. 2021 Mar 30;12(2):e00256-21. doi: 10.1128/mBio.00256-21. mBio. 2021. PMID: 33785615 Free PMC article.
-
Transmissible gastroenteritis virus infection: a vanishing specter.Dtsch Tierarztl Wochenschr. 2006 Apr;113(4):157-9. Dtsch Tierarztl Wochenschr. 2006. PMID: 16716052 Review.
-
An overview of immunological and genetic methods for detecting swine coronaviruses, transmissible gastroenteritis virus, and porcine respiratory coronavirus in tissues.Adv Exp Med Biol. 1997;412:37-46. doi: 10.1007/978-1-4899-1828-4_4. Adv Exp Med Biol. 1997. PMID: 9191988 Review.
Cited by
-
Small GTPase-A Key Role in Host Cell for Coronavirus Infection and a Potential Target for Coronavirus Vaccine Adjuvant Discovery.Viruses. 2022 Sep 14;14(9):2044. doi: 10.3390/v14092044. Viruses. 2022. PMID: 36146850 Free PMC article. Review.
-
Swine coronaviruses (SCoVs) and their emerging threats to swine population, inter-species transmission, exploring the susceptibility of pigs for SARS-CoV-2 and zoonotic concerns.Vet Q. 2022 Dec;42(1):125-147. doi: 10.1080/01652176.2022.2079756. Vet Q. 2022. PMID: 35584308 Free PMC article. Review.
-
The Cell Tropism of Porcine Respiratory Coronavirus for Airway Epithelial Cells Is Determined by the Expression of Porcine Aminopeptidase N.Viruses. 2020 Oct 23;12(11):1211. doi: 10.3390/v12111211. Viruses. 2020. PMID: 33114247 Free PMC article.
-
Coronavirus and the Cytoskeleton of Virus-Infected Cells.Subcell Biochem. 2023;106:333-364. doi: 10.1007/978-3-031-40086-5_12. Subcell Biochem. 2023. PMID: 38159233
-
Deltacoronavirus HKU11, HKU13, PDCoV (HKU15) and HKU17 spike pseudoviruses enter avian DF-1 cells via clathrin-mediated endocytosis in a Rab5-, Rab7- and pH-dependent manner.Vet Res. 2025 Jan 17;56(1):15. doi: 10.1186/s13567-024-01442-3. Vet Res. 2025. PMID: 39825424 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- 2018YFD0500100/National Key Research and Development Program/International
- 31870154/National Natural Science Foundation of China (NSFC)/International
- 31522056/National Natural Science Foundation of China (NSFC)/International
- 61705092/National Natural Science Foundation of China (NSFC)/International
- BK20170194/Natural Science Foundation of Jiangsu Province of China/International
LinkOut - more resources
Full Text Sources

