Epidemiological trends in HCV transmission and prevalence in the Viennese HIV+ population
- PMID: 32017359
- PMCID: PMC7187177
- DOI: 10.1111/liv.14399
Epidemiological trends in HCV transmission and prevalence in the Viennese HIV+ population
Abstract
Background & aims: Human immunodeficiency virus (HIV)/hepatitis C virus (HCV) coinfection is common in people who inject drugs (PWIDs). Recently, 'high-risk' behaviour among men who have sex with men (MSM) has emerged as another main route of HCV transmission. We analysed temporal trends in HCV epidemiology in a cohort of Viennese HIV+ patients.
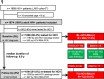
Methods: Hepatitis C virus parameters were recorded at HIV diagnosis (baseline [BL]) and last visit (follow-up [FU]) for all HIV+ patients attending our HIV clinic between January 2014 and December 2016. Proportions of HIV+ patients with anti-HCV(+) and HCV viraemia (HCV-RNA(+)) at BL/FU were assessed and stratified by route of transmission.
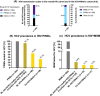
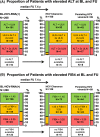
Results: In all, 1806/1874 (96.4%) HIV+ patients were tested for HCV at BL. Anti-HCV(+) was detected in 93.2% (276/296) of PWIDs and in 3.7% (31/839) of MSM. After a median FU of 6.9 years, 1644 (91.0%) patients underwent FU HCV-testing: 167 (90.3%) of PWIDs and 49 (6.7%) of MSM showed anti-HCV(+). Among 208 viraemic HCV-RNA(+) patients at BL, 30 (14.4%) had spontaneously cleared HCV, 76 (36.5%) achieved treatment-induced eradication and 89 (42.8%) remained HCV-RNA(+) at last FU. Among 1433 initially HCV-naive patients, 45 (3.5%) acquired de-novo HCV infection (11.1% PWIDs/80.0% MSM; incidence rate (IR) 0.004%; 95% confidence interval [CI] 0.0%-0.022%) and 14 had HCV reinfections (85.7% PWIDs/14.3% other; IR 0.001%; 95% CI 0.0%-0.018%) during a median FU of 6.7 years (interquartile range 7.4).
Conclusion: Hepatitis C virus testing was successfully implemented in the Viennese HIV(+) patients. Anti-HCV(+) prevalence remained stable in HIV+ PWIDs but almost doubled in HIV+ MSM. De-novo HCV infection occurred mostly in MSM, while HCV reinfections were mainly observed in PWIDs. HCV treatment uptake was suboptimal with 42.8% remaining HCV-RNA(+) at FU.
Keywords: Hepatitis C virus; MSM; PWIDs; epidemiology; human immunodeficiency virus.
© 2020 The Authors. Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
Schmidbauer C received travel support from Gilead and Abbvie. Chromy Dreceived payments for consulting from MSD, Abbvie and Gilead as well as travel support from Abbvie and Gilead. Bauer D received travel support from Gilead and Abbvie. Mandorfer M served as a speaker and/or consultant and/or advisory board member for AbbVie, Bristol‐Myers Squibb, Gilead, and W. L. Gore & Associates and received travel support from AbbVie, Bristol‐Myers Squibb, and Gilead. Simbrunner B received travel support from AbbVie and Gilead. Trauner M served as speaker for BMS, Falk Foundation, Gilead and MSD; advisory boards for Albireo, BiomX, Falk Pharma GmbH, Genfit, Gilead, Intercept, MSD, Novartis, Phenex and Regulus. He further received travel grants from Abbvie, Falk, Gilead and Intercept and research grants from Albireo, Cymabay, Falk, Gilead, Intercept, MSD and Takeda. He is also co‐inventor of patents on the medical use of norUDCA. Gschwantler M received grant support from Abbvie, Gilead and MSD; speaking honoraria from Abbvie, Gilead, Intercept, and MSD; consulting/advisory board fees from Abbvie, Gilead, Intercept and MSD; and travel support from Abbvie and Gilead. Reiberger T received grant support from Abbvie, Boehringer‐Ingelheim, Gilead, MSD, Philips Healthcare, Gore; speaking honoraria from Abbvie, Gilead, Gore, Intercept, Roche, MSD; consulting/advisory board fee from Abbvie, Bayer, Boehringer‐Ingelheim, Gilead, Intercept, MSD, Siemens; and travel support from Boehringer‐Ingelheim, Gilead and Roche. Schmidbauer V, Apata M, Nguyen D, Rieger A, Mayer F, Schmidt R and Holzmann H have nothing to disclose.
Figures

References
-
- Polaris Observatory HCV Collaborators . Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161‐176. - PubMed
-
- Mandorfer M, Schwabl P, Steiner S, Reiberger T, Peck‐Radosavljevic M. Advances in the management of HIV/HCV coinfection. Hepatol Int. 2016;10(3):424‐435. - PubMed
-
- Mandorfer M, Payer BA, Schwabl P, et al. Revisiting liver disease progression in HIV/HCV‐coinfected patients: the influence of vitamin D, insulin resistance, immune status, IL28B and PNPLA3. Liver Int. 2015;35(3):876‐885. - PubMed
-
- Reiberger T, Ferlitsch A, Sieghart W, et al. HIV‐HCV co‐infected patients with low CD4+ cell nadirs are at risk for faster fibrosis progression and portal hypertension. J Viral Hepat. 2010;17(6):400‐409. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

