C-terminal residues of activated protein C light chain contribute to its anticoagulant and cytoprotective activities
- PMID: 32017367
- PMCID: PMC7380734
- DOI: 10.1111/jth.14756
C-terminal residues of activated protein C light chain contribute to its anticoagulant and cytoprotective activities
Abstract
Background: Activated protein C (APC) is an important homeostatic blood coagulation protease that conveys anticoagulant and cytoprotective activities. Proteolytic inactivation of factors Va and VIIIa facilitated by cofactor protein S is responsible for APC's anticoagulant effects, whereas cytoprotective effects of APC involve primarily the endothelial protein C receptor (EPCR), protease activated receptor (PAR)1 and PAR3.
Objective: To date, several binding exosites in the protease domain of APC have been identified that contribute to APC's interaction with its substrates but potential contributions of the C-terminus of the light chain have not been studied in detail.
Methods: Site-directed Ala-scanning mutagenesis of six positively charged residues within G142-L155 was used to characterize their contributions to APC's anticoagulant and cytoprotective activities.
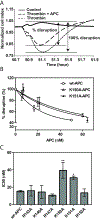
Results and conclusions: K151 was involved in protein S dependent-anticoagulant activity of APC with some contribution of K150. 3D structural analysis supported that these two residues were exposed in an extended protein S binding site on one face of APC. Both K150 and K151 were important for PAR1 and PAR3 cleavage by APC, suggesting that this region may also mediate interactions with PARs. Accordingly, APC's cytoprotective activity as determined by endothelial barrier protection was impaired by Ala substitutions of these residues. Thus, both K150 and K151 are involved in APC's anticoagulant and cytoprotective activities. The differential contribution of K150 relative to K151 for protein S-dependent anticoagulant activity and PAR cleavage highlights that binding exosites for protein S binding and for PAR cleavage in the C-terminal region of APC's light chain overlap.
Keywords: activated protein C; anticoagulant activity; cytoprotective activity; protease activated receptor; protein S.
© 2020 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
CONFLICT OF INTEREST:
TSRI holds intellectual property rights related to some APC variants on which JHG and LOM are listed as inventors. The other authors declare that they have no conflicts of interest.
Figures


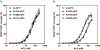
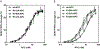

References
-
- O’Donnell JS, O’Sullivan JM, Preston RJS. Advances in understanding the molecular mechanisms that maintain normal haemostasis. Br J Haematol. 2019; 186: 24–36. - PubMed
-
- Dahlback B, Villoutreix BO. Regulation of blood coagulation by the protein C anticoagulant pathway: novel insights into structure-function relationships and molecular recognition. Arterioscler Thromb Vasc Biol. 2005; 25: 1311–20. - PubMed
-
- Wildhagen KC, Lutgens E, Loubele ST, ten Cate H, Nicolaes GA. The structure-function relationship of activated protein C. Lessons from natural and engineered mutations. Thromb Haemost. 2011; 106: 1034–45. - PubMed
-
- Mosnier LO, Griffin JH. Protein C, protein S, thrombomodulin and the endothelial protein C receptor pathways In: Marder VJ, Aird WC, Bennett JS, Schulman S, White GC, Colman RW, eds. Hemostasis and Thrombosis: Basic Principles and Clinical Practice, 6th edn. Philadelphia: Lippincott Williams & Wilkins, 2013, 300–13.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

