Clinical diagnosis and treatment recommendations for ocular toxicities of targeted therapy and immune checkpoint inhibitor therapy
- PMID: 32017399
- PMCID: PMC7049480
- DOI: 10.1111/1759-7714.13327
Clinical diagnosis and treatment recommendations for ocular toxicities of targeted therapy and immune checkpoint inhibitor therapy
Abstract
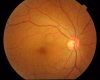
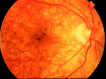
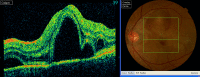
The increased use of targeted therapy and immune checkpoint inhibitors in cancers has brought new hope of survival to patients with advanced tumors. However, increasing numbers of immune-related adverse events (irAEs) of these medications have been reported, affecting almost all human organs including the eye. These adverse effects may affect the entire ocular region, including the eyelid, eye lashes, conjunctiva, cornea, uvea, retina and optic nerve, and have thus far been largely ignored by patients and doctors. In this review, we summarize the characteristics of ocular diseases related to irAEs and advise on how to diagnose and manage these diseases. KEY POINTS: This review will enable clinical oncologists to recognize, diagnose, and manage targeted therapy and immune checkpoint inhibitor-related ocular adverse events.
Keywords: Immune checkpoint inhibitor; immune-related adverse events (irAEs); ocular toxicities; targeted therapy.
© 2020 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures
References
-
- Patnaik A, Kang SP, Rasco D et al Phase I study of Pembrolizumab (MK‐3475; anti‐PD‐1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 2015; 21 (19): 4286–93. - PubMed
-
- Miserocchi E, Cimminiello C, Mazzola M, Russo V, Modorati GM. New‐onset uveitis during CTLA‐4 blockade therapy with ipilimumab in metastatic melanoma patient. Can J Ophthalmol 2015; 50 (1): e2–4. - PubMed
-
- Basilious A, Lloyd JC. Posterior subcapsular cataracts and hypotony secondary to severe pembrolizumab induced uveitis: Case report. Can J Ophthalmol 2016; 51 (1): e4–6. - PubMed
-
- Abu Samra K, Valdes‐Navarro M, Lee S, Swan R, Foster CS, Anesi SD. A case of bilateral uveitis and papillitis in a patient treated with pembrolizumab. Eur J Ophthalmol 2016; 26 (3): e46–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical