Safety Assessment of Benzyne Generation from a Silyl Triflate Precursor
- PMID: 32017583
- PMCID: PMC7193426
- DOI: 10.1021/acs.orglett.0c00267
Safety Assessment of Benzyne Generation from a Silyl Triflate Precursor
Abstract
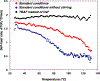
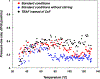
Silyl triflate precursors to benzyne and related intermediates have emerged as valuable synthetic building blocks. However, data addressing the safety of employing these silyl triflate precursors are lacking. We report the calorimetric analysis of a typical Kobayashi procedure for forming and trapping benzyne using a silyl triflate precursor. Our findings suggest that, unlike benzenediazonium carboxylate precursors to benzyne, silyl triflates may be employed under mild conditions without severe concern for runaway reaction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

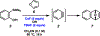
References
-
- For reviews of arynes and related strained intermediates, see:
- Bronner SM; Goetz AE; Garg NK Understanding and Modulating Indolyne Regioselectivities. Synlett 2011, 2599–2604.
- Tadross PM; Stoltz BM A Comprehensive History of Arynes in Natural Product Total Synthesis. Chem. Rev 2012, 112, 3550–3577. - PubMed
- Gampe CM; Carreira EM Arynes and Cyclohexyne in Natural Product Synthesis. Angew. Chem., Int. Ed 2012, 51, 3766–3778. - PubMed
- Dubrovskiy AV; Markina NA; Larock RC Use of Benzynes for the Synthesis of Heterocycles. Org. Biomol. Chem 2013, 11, 191–218. - PubMed
- Hoffmann RW; Suzuki KA “Hot, Energized” Benzyne. Angew. Chem., Int. Ed 2013, 52, 2655–2656. - PubMed
- Goetz AE; Garg NK Enabling the Use of Heterocyclic Arynes in Chemical Synthesis. J. Org. Chem 2014, 79, 846–851. - PubMed
- Yoshida S; Hosoya T The Renaissance and Bright Future of Synthetic Aryne Chemistry. Chem. Lett 2015, 44, 1450–1460.
- Takikawa H; Nishii A; Sakai T; Suzuki K Aryne-Based Strategy in the Total Synthesis of Naturally Occurring Polycyclic Compounds. Chem. Soc. Rev 2018, 47, 8030–8056. - PubMed
- Dhokale RA; Mhaske SB Transition-Metal-Catalyzed Reactions Involving Arynes. Synthesis 2018, 50, 1–16.
-
- For examples of arynes and other strained cyclic alkynes in total synthesis, see:
- Kou KGM; Pflueger JJ; Kiho T; Morrill LC; Fisher EL; Clagg K; Lebold TP; Kisunzu JK; Sarpong R A Benzyne Insertion Approach to Hetisine-Type Diterpenoid Alkaloids: Synthesis of Cossonidine (Davisine). J. Am. Chem. Soc 2018, 140, 8105–8109. - PMC - PubMed
- Goetz AE; Silberstein AL; Corsello MA; Garg NK Concise Enantiospecific Total Synthesis of Tubingensin A. J. Am. Chem. Soc 2014, 136, 3036–3039. - PMC - PubMed
- Neog K; Borah A; Gogoi P Palladium(II)-Catalyzed C–H Bond Activation/C–C and C–O Bond Formation Reaction Cascade: Direct Synthesis of Coumestans. J. Org. Chem 2016, 81, 11971–11977. - PubMed
- Neumeyer M; Kopp J; Brückner R Controlling the Substitution Pattern of Hexasubstituted Naphthalenes by Aryne/Siloxyfuran Diels–Alder Additions: Regio and Stereocontrolled Synthesis of Arizonin C1 Analogs. Eur. J. Org. Chem 2017, 2017, 2883–2915.
- Corsello MA; Kim J; Garg NK Total Synthesis of (–)-Tubingensin B Enabled by the Strategic Use of an Aryne Cyclization. Nat. Chem 2017, 9, 944–949. - PMC - PubMed
- Gampe CM; Carreira EM Total Syntheses of Guanacastepenes N and O. Angew. Chem., Int. Ed 2011, 50, 2962–2965. - PubMed
- Nathel NFF; Shah TK; Bronner SM; Garg NK Total Syntheses of Indolactam Alkaloids (–)-Indolactam V, (–)-Pendolmycin, (–)-Lyngbyatoxin A, and (–)-Teleocidin A-2. Chem. Sci 2014, 5, 2184–2190. - PMC - PubMed
-
- Surry DS; Buchwald SL Biaryl Phosphane Ligands in Palladium-Catalyzed Amination. Angew. Chem., Int. Ed 2008, 47, 6338–6361. - PMC - PubMed
- Mauger CC; Mignani GA An Efficient and Safe Procedure for the Large-Scale Pd-Catalyzed Hydrazonation of Aromatic Chlorides Using Buchwald Technology. Org. Process Res. Dev 2004, 8, 1065–1071.
-
- Carroll FI; Robinson TP; Brieaddy LE; Atkinson RN; Mascarella SW; Damaj MI; Martin BR; Navarro HA Synthesis and Nicotinic Acetylcholine Receptor Binding Properties of Bridged and Fused Ring Analogues of Epibatidine. J. Med. Chem 2007, 50, 6383–6391. - PubMed
- Kametani T; Kigasawa K; Hiragi M; Wagatsuma N; Uryu T; Araki K Syntheses of Heterocyclic Compounds. 509. Syntheses of Analgesics. 34. Synthesis of 3-Hydroxy-N-Cyclopropylmethyl-9-Azamorphinan. J. Med. Chem 1973, 16, 301–303. - PubMed
- Heindel ND; Fives WP; Lemke TF; Rowe JE; Snady HW Synthesis, Transformation, and General Pharmacologic Activity in 1,4-Benzodiazepine-3,5-diones. J. Med. Chem 1971, 14, 1233–1235. - PubMed
- Bell MR; Zalay AW; Oesterlin R; Schane P; Potts G Basic Ethers of 1-(p-Hydroxyphenyl)-2-Phenyl-1,2,3,4-Tetrahydroquinoline and 1-(p-Hydroxyphenyl)-2-Phenyl Indole. Antifertility Agents. J. Med. Chem 1970, 13, 664–668. - PubMed
- Willis PG; Pavlova OA; Chefer SI; Vaupel DB; Mukhin AG; Horti AG Synthesis and Structure-Activity Relationship of a Novel Series of Aminoalkylindoles with Potential of Imaging the Neuronal Cannabinoid Receptor by Positron Emission Tomography. J. Med. Chem 2005, 48, 5813–5822. - PubMed
- Jacob P III; Shulgin AT Sulfur Analogues of Psychotomimetic Agents. Monothio Analogues of Mescaline and Isomescaline. J. Med. Chem 1981, 24, 1348–1353. - PubMed
- Coe JW; Brooks PR; Wirtz MC; Bashore CG; Bianco KE; Vetelino MG; Arnold EP; Lebel LA; Fox CB; Tingley FD III; Schulz DW; Davis TI; Sands SB; Mansbach RS; Rollema H; O’Neill BT 3,5-Bicyclic Aryl Piperidines: A Novel Class of α4β2 Neuronal Nicotinic Receptor Partial Agonists for Smoking Cessation. Bioorg. Med. Chem. Lett 2005, 15, 4889–4897. - PubMed
-
-
Lin JB; Shah TK; Goetz AE; Garg NK; Houk KN Conjugated Trimeric Scaffolds Accessible from Indolyne Cyclotrimerizations: Synthesis, Structure, and Electronic Properties. J. Am. Chem. Soc 2017, 139, 10447–10455.Darzi ER; Barber JS; Garg NK Cyclic Alkyne Approach to Heteroatom-Containing Polycyclic Aromatic Hydrocarbon Scaffolds. Angew. Chem., Int. Ed 2019, 58, 9419–9424.For a review of arynes in the syntheses of polycyclic aromatic hydrocarbon materials, see: Pérez D; Peña D; Guitián E Aryne Cycloaddition Reactions in the Synthesis of Large Polycyclic Aromatic Compounds. Eur. J. Org. Chem 2013, 2013, 5981–6013. Suh SE; Barros SA; Chenoweth DM Triple Aryne–Tetrazine Reaction Enabling Rapid Access to a New Class of Polyaromatic Heterocycles. Chem. Sci 2015, 6, 5128–5132. Sulleiro MV; Quiroga S; Peña D; Guitián E; Criado A; Prato M Microwave-Induced Covalent Functionalization of Few-Layer Graphene with Arynes under Solvent-Free Conditions. Chem. Commun 2018, 54, 2086–2089.Criado A; Vizuete M; Gómez-Escalonilla MJ; García-Rodriguez S; Fierro JLG; Cobas A; Peña D; Guitián E; Langa F Efficient Cycloaddition of Arynes to Carbon Nanotubes under Microwave Irradiation. Carbon 2013, 63, 140–148.Criado A; Gómez-Escalonilla MJ; Fierro JLG; Urbina A; Peña D; Guitián E; Langa F Cycloaddition of Benzyne to SWCNT: Towards CNT-Based Paddle Wheels. Chem. Commun 2010, 46, 7028–7030.Chronopoulos D; Karousis N; Ichihashi T; Yudasaka M; Iijima S; Tagmatarchis N Benzyne Cycloaddition onto Carbon Nanohorns. Nanoscale 2013, 5, 6388–6394.García D; Rodríguez-Pérez L; Herranz MA; Peña D; Guitian E; Bailey S; Al-Galiby Q; Noori M; Lambert CJ; Pérez D; Martín N A C60-Aryne Building Block: Synthesis of a Hybrid All-Carbon Nanostructure. Chem. Commun 2016, 52, 6677–6680.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

