Plasma Circulating Tumor HPV DNA for the Surveillance of Cancer Recurrence in HPV-Associated Oropharyngeal Cancer
- PMID: 32017652
- PMCID: PMC7106982
- DOI: 10.1200/JCO.19.02444
Plasma Circulating Tumor HPV DNA for the Surveillance of Cancer Recurrence in HPV-Associated Oropharyngeal Cancer
Erratum in
-
Errata.J Clin Oncol. 2020 Oct 20;38(30):3579. doi: 10.1200/JCO.20.02655. J Clin Oncol. 2020. PMID: 33058748 Free PMC article. No abstract available.
-
Erratum: Plasma Circulating Tumor HPV DNA for the Surveillance of Cancer Recurrence in HPV-Associated Oropharyngeal Cancer.J Clin Oncol. 2023 Sep 20;41(27):4449. doi: 10.1200/JCO.23.01228. Epub 2023 Aug 4. J Clin Oncol. 2023. PMID: 37540822 Free PMC article. No abstract available.
Abstract
Purpose: Plasma circulating tumor human papillomavirus DNA (ctHPVDNA) is a sensitive and specific biomarker of human papillomavirus (HPV)-associated oropharyngeal squamous cell carcinoma (OPSCC). We investigated whether longitudinal monitoring of ctHPVDNA during post-treatment surveillance could accurately detect clinical disease recurrence.
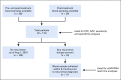
Methods and materials: A prospective biomarker clinical trial was conducted among patients with nonmetastatic HPV-associated (p16-positive) OPSCC. All patients were treated with curative-intent chemoradiotherapy (CRT). Patients underwent a 3-month post-CRT positron emission tomography/computed tomography scan and were thereafter clinically evaluated every 2-4 months (years 1-2), then every 6 months (years 3-5). Chest imaging was performed every 6 months. Blood specimens were collected every 6-9 months for analysis of plasma ctHPVDNA using a multianalyte digital polymerase chain reaction assay. The primary endpoint was to estimate the negative predictive value (NPV) and positive predictive value (PPV) of ctHPVDNA surveillance.
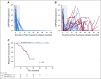
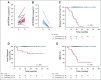
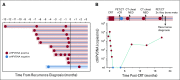
Results: One hundred fifteen patients were enrolled, and 1,006 blood samples were analyzed. After a median follow-up time of 23 months (range, 6.1-54.7 months), 15 patients (13%) developed disease recurrence. Eighty-seven patients had undetectable ctHPVDNA at all post-treatment time points, and none developed recurrence (NPV, 100%; 95% CI, 96% to 100%). Twenty-eight patients developed a positive ctHPVDNA during post-treatment surveillance, 15 of whom were diagnosed with biopsy-proven recurrence. Sixteen patients had 2 consecutively positive ctHPVDNA blood tests, 15 of whom developed biopsy-proven recurrence. Two consecutively positive ctHPVDNA blood tests had a PPV of 94% (95% CI, 70% to 99%). Median lead time between ctHPVDNA positivity and biopsy-proven recurrence was 3.9 months (range, 0.37-12.9 months).
Conclusion: Detection of ctHPVDNA in two consecutive plasma samples during post-treatment surveillance has high PPV and NPV for identifying disease recurrence in patients with HPV-associated oropharyngeal cancer and may facilitate earlier initiation of salvage therapy.
Trial registration: ClinicalTrials.gov NCT02281955 NCT03077243 NCT03161821.
Figures
References
-
- Centers for Disease Control and Prevention HPV-Associated Cancer Statistics. . https://www.cdc.gov/cancer/dcpc/data/index.htm
-
- Chera BS, Amdur RJ, Tepper J, et al. Phase 2 trial of de-intensified chemoradiation therapy for favorable-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2015;93:976–985. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

