Ketamine: The final frontier or another depressing end?
- PMID: 32017978
- PMCID: PMC7127859
- DOI: 10.1016/j.bbr.2020.112508
Ketamine: The final frontier or another depressing end?
Abstract
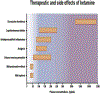
Two decades ago, the observation of a rapid and sustained antidepressant response after ketamine administration provided an exciting new avenue in the search for more effective therapeutics for the treatment of clinical depression. Research elucidating the mechanism(s) underlying ketamine's antidepressant properties has led to the development of several hypotheses, including that of disinhibition of excitatory glutamate neurons via blockade of N-methyl-d-aspartate (NMDA) receptors. Although the prominent understanding has been that ketamine's mode of action is mediated solely via the NMDA receptor, this view has been challenged by reports implicating other glutamate receptors such as AMPA, and other neurotransmitter systems such as serotonin and opioids in the antidepressant response. The recent approval of esketamine (Spravato™) for the treatment of depression has sparked a resurgence of interest for a deeper understanding of the mechanism(s) underlying ketamine's actions and safe therapeutic use. This review aims to present our current knowledge on both NMDA and non-NMDA mechanisms implicated in ketamine's response, and addresses the controversy surrounding the antidepressant role and potency of its stereoisomers and metabolites. There is much that remains to be known about our understanding of ketamine's antidepressant properties; and although the arrival of esketamine has been received with great enthusiasm, it is now more important than ever that its mechanisms of action be fully delineated, and both the short- and long-term neurobiological/functional consequences of its treatment be thoroughly characterized.
Keywords: Antidepressant mechanism; Depression; Ketamine; Major Depressive Disorder; NMDA; Non-NMDA mechanism; Rapid antidepressant; Spravato.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures
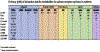


Similar articles
-
Overlap in the neural circuitry and molecular mechanisms underlying ketamine abuse and its use as an antidepressant.Behav Brain Res. 2020 Apr 20;384:112548. doi: 10.1016/j.bbr.2020.112548. Epub 2020 Feb 13. Behav Brain Res. 2020. PMID: 32061748 Free PMC article. Review.
-
Two cellular hypotheses explaining the initiation of ketamine's antidepressant actions: Direct inhibition and disinhibition.Neuropharmacology. 2016 Jan;100:17-26. doi: 10.1016/j.neuropharm.2015.07.028. Epub 2015 Jul 26. Neuropharmacology. 2016. PMID: 26211972 Review.
-
An extension of hypotheses regarding rapid-acting, treatment-refractory, and conventional antidepressant activity of dextromethorphan and dextrorphan.Med Hypotheses. 2012 Jun;78(6):693-702. doi: 10.1016/j.mehy.2012.02.012. Epub 2012 Mar 7. Med Hypotheses. 2012. PMID: 22401777
-
What is the mechanism of Ketamine's rapid-onset antidepressant effect? A concise overview of the surprisingly large number of possibilities.J Clin Pharm Ther. 2017 Apr;42(2):147-154. doi: 10.1111/jcpt.12497. Epub 2017 Jan 22. J Clin Pharm Ther. 2017. PMID: 28111761 Review.
-
A review of ketamine in affective disorders: current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action.J Affect Disord. 2014 Mar;156:24-35. doi: 10.1016/j.jad.2013.11.014. Epub 2013 Dec 10. J Affect Disord. 2014. PMID: 24388038 Review.
Cited by
-
Ketamine beyond anesthesia: Antidepressant effects and abuse potential.Behav Brain Res. 2020 Sep 15;394:112841. doi: 10.1016/j.bbr.2020.112841. Epub 2020 Jul 31. Behav Brain Res. 2020. PMID: 32739287 Free PMC article. No abstract available.
-
Effects of recurrent ketamine exposure on brain histopathology in juvenile rats.Turk J Med Sci. 2023 Feb;53(1):19-28. doi: 10.55730/1300-0144.5554. Epub 2023 Feb 22. Turk J Med Sci. 2023. PMID: 36945933 Free PMC article.
-
Habenula bibliometrics: Thematic development and research fronts of a resurgent field.Front Integr Neurosci. 2022 Aug 3;16:949162. doi: 10.3389/fnint.2022.949162. eCollection 2022. Front Integr Neurosci. 2022. PMID: 35990593 Free PMC article.
-
Ketamine Use in Child and Adolescent Psychiatry: Emerging Data in Treatment-Resistant Depression, Insights from Adults, and Future Directions.Curr Psychiatry Rep. 2023 Aug;25(8):337-344. doi: 10.1007/s11920-023-01432-w. Epub 2023 Jun 30. Curr Psychiatry Rep. 2023. PMID: 37389787 Review.
-
Targeting inflammation in depression: Ketamine as an anti-inflammatory antidepressant in psychiatric emergency.Brain Behav Immun Health. 2021 Nov 10;18:100383. doi: 10.1016/j.bbih.2021.100383. eCollection 2021 Dec. Brain Behav Immun Health. 2021. PMID: 34849492 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

