Reorganization of rich-clubs in functional brain networks during propofol-induced unconsciousness and natural sleep
- PMID: 32018124
- PMCID: PMC6997627
- DOI: 10.1016/j.nicl.2020.102188
Reorganization of rich-clubs in functional brain networks during propofol-induced unconsciousness and natural sleep
Abstract
Background: General anesthesia (GA) provides an invaluable experimental tool to understand the essential neural mechanisms underlying consciousness. Previous neuroimaging studies have shown the functional integration and segregation of brain functional networks during anesthetic-induced alteration of consciousness. However, the organization pattern of hubs in functional brain networks remains unclear. Moreover, comparisons with the well-characterized physiological unconsciousness can help us understand the neural mechanisms of anesthetic-induced unconsciousness.
Methods: Resting-state functional magnetic resonance imaging was performed during wakefulness, mild propofol-induced sedation (m-PIS), and deep PIS (d-PIS) with clinical unconsciousness on 8 healthy volunteers and wakefulness and natural sleep on 9 age- and sex-matched healthy volunteers. Large-scale functional brain networks of each volunteer were constructed based on 160 regions of interest. Then, rich-club organizations in brain functional networks and nodal properties (nodal strength and efficiency) were assessed and analyzed among the different states and groups.
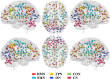
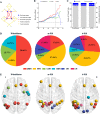
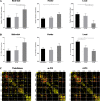
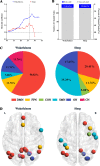
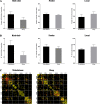
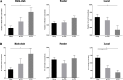
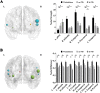
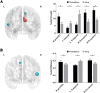
Results: Rich-clubs in the functional brain networks were reorganized during alteration of consciousness induced by propofol. Firstly, rich-club nodes were switched from the posterior cingulate cortex (PCC), angular gyrus, and anterior and middle insula to the inferior parietal lobule (IPL), inferior parietal sulcus (IPS), and cerebellum. When sedation was deepened to unconsciousness, the rich-club nodes were switched to the occipital and angular gyrus. These results suggest that the rich-club nodes were switched among the high-order cognitive function networks (default mode network [DMN] and fronto-parietal network [FPN]), sensory networks (occipital network [ON]), and cerebellum network (CN) from consciousness (wakefulness) to propofol-induced unconsciousness. At the same time, compared with wakefulness, local connections were switched to rich-club connections during propofol-induced unconsciousness, suggesting a strengthening of the overall information commutation of networks. Nodal efficiency of the anterior and middle insula and ventral frontal cortex was significantly decreased. Additionally, from wakefulness to natural sleep, a similar pattern of rich-club reorganization with propofol-induced unconsciousness was observed: rich-club nodes were switched from the DMN (including precuneus and PCC) to the sensorimotor network (SMN, including part of the frontal and temporal gyrus). Compared with natural sleep, nodal efficiency of the insula, frontal gyrus, PCC, and cerebellum significantly decreased during propofol-induced unconsciousness.
Conclusions: Our study demonstrated that the rich-club reorganization in functional brain networks is characterized by switching of rich-club nodes between the high-order cognitive and sensory and motor networks during propofol-induced alteration of consciousness and natural sleep. These findings will help understand the common neurological mechanism of pharmacological and physiological unconsciousness.
Keywords: Brain network; Natural sleep; Propofol-induced sedation; Resting-state functional magnetic resonance images (rs-fMRI); Rich-club organization.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing interests.
Figures
Similar articles
-
Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness.Anesthesiology. 2010 Nov;113(5):1038-53. doi: 10.1097/ALN.0b013e3181f697f5. Anesthesiology. 2010. PMID: 20885292
-
Cortical functional connectivity indexes arousal state during sleep and anesthesia.Neuroimage. 2020 May 1;211:116627. doi: 10.1016/j.neuroimage.2020.116627. Epub 2020 Feb 8. Neuroimage. 2020. PMID: 32045640 Free PMC article.
-
Meso-scale reorganization of local-global brain networks under mild sedation of propofol anesthesia.Neuroimage. 2024 Aug 15;297:120744. doi: 10.1016/j.neuroimage.2024.120744. Epub 2024 Jul 20. Neuroimage. 2024. PMID: 39033791
-
The structural and functional connectivity of the posterior cingulate cortex: comparison between deterministic and probabilistic tractography for the investigation of structure-function relationships.Neuroimage. 2014 Nov 15;102 Pt 1:118-27. doi: 10.1016/j.neuroimage.2013.12.022. Epub 2013 Dec 21. Neuroimage. 2014. PMID: 24365673 Review.
-
No cognitive processing in the unconscious, anesthetic-like, state of sleep.J Comp Neurol. 2021 Feb;529(3):524-538. doi: 10.1002/cne.24963. Epub 2020 Jun 30. J Comp Neurol. 2021. PMID: 32472571 Free PMC article. Review.
Cited by
-
Take-Home Video Shortens the Time to First Ambulation in Patients With Inguinal Hernia Repair Under General Anesthesia: A Retrospective Observational Study.Front Med (Lausanne). 2022 Jun 29;9:848280. doi: 10.3389/fmed.2022.848280. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35847805 Free PMC article.
-
Functional networks in prolonged disorders of consciousness.Front Neurosci. 2023 Feb 17;17:1113695. doi: 10.3389/fnins.2023.1113695. eCollection 2023. Front Neurosci. 2023. PMID: 36875660 Free PMC article. Review.
-
Toward asleep DBS: cortico-basal ganglia spectral and coherence activity during interleaved propofol/ketamine sedation mimics NREM/REM sleep activity.NPJ Parkinsons Dis. 2021 Aug 2;7(1):67. doi: 10.1038/s41531-021-00211-9. NPJ Parkinsons Dis. 2021. PMID: 34341348 Free PMC article.
-
Consciousness and complexity: a consilience of evidence.Neurosci Conscious. 2021 Aug 30;2021(2):niab023. doi: 10.1093/nc/niab023. eCollection 2021. Neurosci Conscious. 2021. PMID: 38496724 Free PMC article. Review.
-
The effects of the general anesthetic sevoflurane on neurotransmission: an experimental and computational study.Sci Rep. 2021 Feb 22;11(1):4335. doi: 10.1038/s41598-021-83714-y. Sci Rep. 2021. PMID: 33619298 Free PMC article.
References
-
- Absalom A.R., Mani V., Smet T., Struys M.M.R.F. Pharmacokinetic models for propofol-defining and illuminating the devil in the detail. Br. J. Anaesth. 2009;103(1):26–37. - PubMed
-
- Adapa R.M., Axell R.G., Mangat J.S., Carpenter T.A., Absalom A.R. Safety and performance of TCI pumps in a magnetic resonance imaging environment. Anaesthesia. 2012;67(1):33–39. - PubMed
-
- Albert, Jeong Barabasi Error and attack tolerance of complex networks. Nature. 2000;406(6794):378–382. - PubMed
-
- Alkire M.T., Miller J. General anesthesia and the neural correlates of consciousness. Prog. Brain Res. 2005;150:229–244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

