Estradiol-17β-Induced Changes in the Porcine Endometrial Transcriptome In Vivo
- PMID: 32019139
- PMCID: PMC7037416
- DOI: 10.3390/ijms21030890
Estradiol-17β-Induced Changes in the Porcine Endometrial Transcriptome In Vivo
Abstract
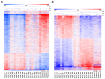
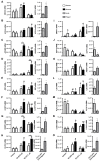
Estradiol-17β (E2) is a key hormone regulating reproductive functions in females. In pigs, E2, as the main conceptus signal, initiates processes resulting in prolonged corpus luteum function, embryo development, and implantation. During early pregnancy the endometrium undergoes morphological and physiological transitions that are tightly related to transcriptome changes. Recently, however, the importance of E2 as a primary conceptus signal in the pig has been questionable. Thus, the aim of the present study was to determine the effects of E2 on the porcine endometrial transcriptome in vivo and to compare these effects with transcriptome profiles on day 12 of pregnancy. Microarray analysis revealed differentially expressed genes (DEGs) in response to E2 with overrepresented functional terms related to secretive functions, extracellular vesicles, cell adhesion, proliferation and differentiation, tissue rearrangements, immune response, lipid metabolism, and many others. Numerous common DEGs and processes for the endometrium on day 12 of pregnancy and E2-treated endometrium were identified. In summary, the present study is the first evidence for the effect of E2 on transcriptome profiles in porcine endometrium in vivo in the period corresponding to the maternal recognition of pregnancy. The presented results provide a valuable resource for further targeted studies considering genes and pathways regulated by conceptus-derived estrogens and their role in pregnancy establishment.
Keywords: Sus scrofa; early pregnancy; endometrium; estradiol-17β; pig; transcriptome profiling.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





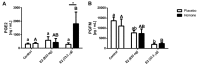
Similar articles
-
Synergistic action of estradiol and PGE2 on endometrial transcriptome in vivo resembles pregnancy effects better than estradiol alone†.Biol Reprod. 2021 Apr 1;104(4):818-834. doi: 10.1093/biolre/ioaa230. Biol Reprod. 2021. PMID: 33354726
-
Transcriptome changes in the porcine endometrium during the preattachment phase.Biol Reprod. 2013 Dec 12;89(6):134. doi: 10.1095/biolreprod.113.112177. Print 2013 Dec. Biol Reprod. 2013. PMID: 24174570
-
Transcriptomic analysis of interferon-γ-regulated genes in endometrial explants and their possible role in regulating maternal endometrial immunity during the implantation period in pigs, a true epitheliochorial placentation species.Theriogenology. 2020 Oct 1;155:114-124. doi: 10.1016/j.theriogenology.2020.05.045. Epub 2020 Jun 22. Theriogenology. 2020. PMID: 32659448
-
Embryo-maternal dialogue during pregnancy establishment and implantation in the pig.Mol Reprod Dev. 2017 Sep;84(9):842-855. doi: 10.1002/mrd.22835. Epub 2017 Jun 19. Mol Reprod Dev. 2017. PMID: 28628266 Review.
-
Biochemical aspects of conceptus--endometrial interactions.J Exp Zool. 1983 Nov;228(2):373-83. doi: 10.1002/jez.1402280220. J Exp Zool. 1983. PMID: 6363610 Review.
Cited by
-
Sexual Dimorphism in Innate Immunity: The Role of Sex Hormones and Epigenetics.Front Immunol. 2021 Jan 21;11:604000. doi: 10.3389/fimmu.2020.604000. eCollection 2020. Front Immunol. 2021. PMID: 33584674 Free PMC article. Review.
-
Identification of Candidate Salivary, Urinary and Serum Metabolic Biomarkers for High Litter Size Potential in Sows (Sus scrofa).Metabolites. 2022 Oct 30;12(11):1045. doi: 10.3390/metabo12111045. Metabolites. 2022. PMID: 36355128 Free PMC article.
-
Embryo-Maternal Interactions Underlying Reproduction in Mammals.Int J Mol Sci. 2020 Jul 10;21(14):4872. doi: 10.3390/ijms21144872. Int J Mol Sci. 2020. PMID: 32664189 Free PMC article.
-
MicroRNA participates in embryo implantation by modulating endometrial tolerance in sows during peri-implantation period.Front Endocrinol (Lausanne). 2025 Aug 5;16:1555636. doi: 10.3389/fendo.2025.1555636. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40838210 Free PMC article. Review.
-
Revisiting the Transcriptome Landscape of Pig Embryo Implantation Site at Single-Cell Resolution.Front Cell Dev Biol. 2022 May 4;10:796358. doi: 10.3389/fcell.2022.796358. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35602598 Free PMC article.
References
-
- Pope W.F., First N.L. Factors affecting the survival of pig embryos. Theriogenology. 1985;23:91–105. doi: 10.1016/0093-691X(85)90075-5. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

