High-Mobility Group Box 1 Protein Signaling in Painful Diabetic Neuropathy
- PMID: 32019145
- PMCID: PMC7036925
- DOI: 10.3390/ijms21030881
High-Mobility Group Box 1 Protein Signaling in Painful Diabetic Neuropathy
Abstract
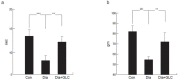
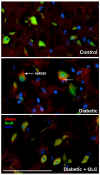
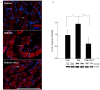
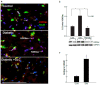
Diabetes is a global epidemic and more than 50% diabetic patients are also diagnosed with neuropathy, which greatly affects the quality of life of the patients. Available treatments are not always successful due to the limited efficacy and complications, such as addiction and dependency. Studies have implicated that high mobility group box1 (HMGB1) protein plays a crucial role in neuroinflammation and the development of neuropathic conditions. HMGB1 is a proinflammatory cytokine that can be released from necrotic cells in passive form or in response to inflammatory signals as an active form. HMGB1 is the ligand for the receptor for advanced glycation end products (RAGE), and toll-like receptors, (TLR)-2 and TLR4, which also indirectly activates C-X-C chemokine receptor type 4 (CXCR4). We investigated whether blocking of HMGB1 can reduce pain and inflammation in diabetic neuropathic animals to further understand the role of HMGB1 in diabetic neuropathy. Type 2 diabetic rats and mice were treated with natural inhibitor of HMGB1, glycyrrhizin (GLC) for five days/week for four weeks at a dose of 50 mg/kg per day by intraperitoneal injection. The animals were divided into three categories: naïve control, diabetic alone, diabetic with GLC treatment. All of the behavioral analyses were conducted before and after the treatment. The expression of inflammatory markers and changes in histone acetylation in the peripheral nervous system were measured by immunohistochemistry and Western blot analysis after the completion of the treatment. Our study revealed that TLR4, HMGB1, CXCR4, and Nod-like receptor protein 3 (NLRP3) levels were increased in the spinal and dorsal root ganglia (DRG) neurons of Type 2 diabetic mice and rats with painful neuropathy. GLC treatment inhibited the increases in TLR4, NLRP3, and CXCR4 expressions and improved the mechanical and thermal pain threshold in these animals. Immunohistochemical studies revealed that hyperglycemia mediated inflammation influenced HMGB1 acetylation and its release from the neurons. It also altered histone 3 acetylation in the microglial cells. The inhibition of HMGB1 by GLC prevented the release of HMGB1 as well as H3K9 acetylation. These findings indicate that the interruption of HMGB1 mediated inflammation could ameliorate diabetic neuropathy and might exhibit a unique target for the treatment.
Keywords: Diabetes; glycyrrhizin; high mobility group box1 (HMGB1); inflammation; neuropathy.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
High-Mobility Group Box-1 Modulates the Expression of Inflammatory and Angiogenic Signaling Pathways in Diabetic Retina.Curr Eye Res. 2015;40(11):1141-52. doi: 10.3109/02713683.2014.982829. Epub 2014 Dec 11. Curr Eye Res. 2015. PMID: 25495026
-
High Mobility Group Box1 Inhibitor Glycyrrhizic Acid Attenuates Kidney Injury in Streptozotocin-Induced Diabetic Rats.Kidney Blood Press Res. 2017;42(5):894-904. doi: 10.1159/000485045. Epub 2017 Nov 15. Kidney Blood Press Res. 2017. PMID: 29241180
-
Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-kB pathway.Exp Cell Res. 2018 Aug 1;369(1):112-119. doi: 10.1016/j.yexcr.2018.05.012. Epub 2018 May 12. Exp Cell Res. 2018. PMID: 29763588
-
Expatiating the molecular approaches of HMGB1 in diabetes mellitus: Highlighting signalling pathways via RAGE and TLRs.Mol Biol Rep. 2021 Feb;48(2):1869-1881. doi: 10.1007/s11033-020-06130-x. Epub 2021 Jan 21. Mol Biol Rep. 2021. PMID: 33479829 Review.
-
Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1).Angiogenesis. 2008;11(1):91-9. doi: 10.1007/s10456-008-9093-5. Epub 2008 Feb 9. Angiogenesis. 2008. PMID: 18264787 Review.
Cited by
-
Novel Mechanism for Memantine in Attenuating Diabetic Neuropathic Pain in Mice via Downregulating the Spinal HMGB1/TRL4/NF-kB Inflammatory Axis.Pharmaceuticals (Basel). 2021 Apr 1;14(4):307. doi: 10.3390/ph14040307. Pharmaceuticals (Basel). 2021. PMID: 33915770 Free PMC article.
-
Lysophosphatidic Acid Receptor 1- and 3-Mediated Hyperalgesia and Hypoalgesia in Diabetic Neuropathic Pain Models in Mice.Cells. 2020 Aug 16;9(8):1906. doi: 10.3390/cells9081906. Cells. 2020. PMID: 32824296 Free PMC article.
-
Clinical Pathobiochemistry of Vitamin B12 Deficiency: Improving Our Understanding by Exploring Novel Mechanisms with a Focus on Diabetic Neuropathy.Nutrients. 2023 Jun 1;15(11):2597. doi: 10.3390/nu15112597. Nutrients. 2023. PMID: 37299560 Free PMC article. Review.
-
CXCR4/CX43 Regulate Diabetic Neuropathic Pain via Intercellular Interactions between Activated Neurons and Dysfunctional Astrocytes during Late Phase of Diabetes in Rats and the Effects of Antioxidant N-Acetyl-L-Cysteine.Oxid Med Cell Longev. 2022 Jun 28;2022:8547563. doi: 10.1155/2022/8547563. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35799894 Free PMC article.
-
Peripheral Neuropathy Phenotyping in Rat Models of Type 2 Diabetes Mellitus: Evaluating Uptake of the Neurodiab Guidelines and Identifying Future Directions.Diabetes Metab J. 2022 Mar;46(2):198-221. doi: 10.4093/dmj.2021.0347. Epub 2022 Mar 24. Diabetes Metab J. 2022. PMID: 35385634 Free PMC article. Review.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

