In Vitro Evaluation of Different Prebiotics on the Modulation of Gut Microbiota Composition and Function in Morbid Obese and Normal-Weight Subjects
- PMID: 32019174
- PMCID: PMC7038051
- DOI: 10.3390/ijms21030906
In Vitro Evaluation of Different Prebiotics on the Modulation of Gut Microbiota Composition and Function in Morbid Obese and Normal-Weight Subjects
Abstract
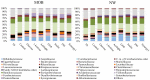
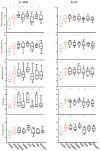
The gut microbiota remains relatively stable during adulthood; however, certain intrinsic and environmental factors can lead to microbiota dysbiosis. Its restoration towards a healthy condition using best-suited prebiotics requires previous development of in vitro models for evaluating their functionality. Herein, we carried out fecal cultures with microbiota from healthy normal-weight and morbid obese adults. Cultures were supplemented with different inulin-type fructans (1-kestose, Actilight, P95, Synergy1 and Inulin) and a galactooligosaccharide. Their impact on the gut microbiota was assessed by monitoring gas production and evaluating changes in the microbiota composition (qPCR and 16S rRNA gene profiling) and metabolic activity (gas chromatography). Additionally, the effect on the bifidobacterial species was assessed (ITS-sequencing). Moreover, the functionality of the microbiota before and after prebiotic-modulation was determined in an in vitro model of interaction with an intestinal cell line. In general, 1-kestose was the compound showing the largest effects. The modulation with prebiotics led to significant increases in the Bacteroides group and Faecalibacterium in obese subjects, whereas in normal-weight individuals, substantial rises in Bifidobacterium and Faecalibacterium were appreciated. Notably, the results obtained showed differences in the responses among the tested compounds but also among the studied human populations, indicating the need for developing population-specific products.
Keywords: HT29; RTCA; SCFA; bifidobacterial-ITS; functionality; gas production; in vitro model; microbiota; obesity; prebiotics.
Conflict of interest statement
Takumi Tochio and Katsuaki Hirano are employees at the company β- Food Sciences.
Figures

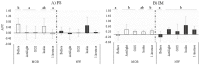
Similar articles
-
Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: a randomised, double-blind, placebo-controlled, cross-over, human intervention study.Br J Nutr. 2018 Jan;119(2):176-189. doi: 10.1017/S0007114517003440. Epub 2018 Jan 8. Br J Nutr. 2018. PMID: 29307330 Clinical Trial.
-
Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels.Gut Microbes. 2019;10(3):334-357. doi: 10.1080/19490976.2018.1526583. Epub 2018 Nov 5. Gut Microbes. 2019. PMID: 30395776 Free PMC article.
-
Prebiotic supplementation over a cold season and during antibiotic treatment specifically modulates the gut microbiota composition of 3-6 year-old children.Benef Microbes. 2019 Apr 19;10(3):253-263. doi: 10.3920/BM2018.0116. Epub 2019 Feb 19. Benef Microbes. 2019. PMID: 30776899 Clinical Trial.
-
Effect of fructans, prebiotics and fibres on the human gut microbiome assessed by 16S rRNA-based approaches: a review.Benef Microbes. 2020 Mar 27;11(2):101-129. doi: 10.3920/BM2019.0082. Epub 2020 Feb 19. Benef Microbes. 2020. PMID: 32073295 Review.
-
Prebiotic effects: metabolic and health benefits.Br J Nutr. 2010 Aug;104 Suppl 2:S1-63. doi: 10.1017/S0007114510003363. Br J Nutr. 2010. PMID: 20920376 Review.
Cited by
-
Probiotic-Induced Modulation of Microbiota Composition and Antibiotic Resistance Genes Load, an In Vitro Assessment.Int J Mol Sci. 2024 Jan 13;25(2):1003. doi: 10.3390/ijms25021003. Int J Mol Sci. 2024. PMID: 38256076 Free PMC article.
-
Potential Prebiotic Effects of Artemisia capillaris-Derived Transglycosylated Product.Foods. 2024 Oct 14;13(20):3267. doi: 10.3390/foods13203267. Foods. 2024. PMID: 39456329 Free PMC article.
-
Comparative analysis of prebiotic effects of four oligosaccharides using in vitro gut model: digestibility, microbiome, and metabolome changes.FEMS Microbiol Ecol. 2023 Jan 24;99(2):fiad002. doi: 10.1093/femsec/fiad002. FEMS Microbiol Ecol. 2023. PMID: 36623850 Free PMC article.
-
In vitro Selection of Probiotics for Microbiota Modulation in Normal-Weight and Severely Obese Individuals: Focus on Gas Production and Interaction With Intestinal Epithelial Cells.Front Microbiol. 2021 Feb 9;12:630572. doi: 10.3389/fmicb.2021.630572. eCollection 2021. Front Microbiol. 2021. PMID: 33633711 Free PMC article.
-
Propionate and butyrate counteract renal damage and progression to chronic kidney disease.Nephrol Dial Transplant. 2024 Dec 20;40(1):133-150. doi: 10.1093/ndt/gfae118. Nephrol Dial Transplant. 2024. PMID: 38794880 Free PMC article.
References
-
- Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. - DOI - PubMed
-
- Vernazza C.L., Rabiu A.R., Gibson G.R. Human Colonic Microbiology and the Role of Dietary Intervention: Introduction to Prebiotics. In: Gibson G.R., Rastall R.A., editors. Prebiotics: Development & Application. John Wiley & Sons, Ltd.; Chichester, UK: 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

