Binder-Free V2O5 Cathode for High Energy Density Rechargeable Aluminum-Ion Batteries
- PMID: 32019197
- PMCID: PMC7075190
- DOI: 10.3390/nano10020247
Binder-Free V2O5 Cathode for High Energy Density Rechargeable Aluminum-Ion Batteries
Abstract
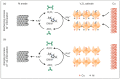
Nowadays, research on electrochemical storage systems moves into the direction of post-lithium-ion batteries, such as aluminum-ion batteries, and the exploration of suitable materials for such batteries. Vanadium pentoxide (V2O5) is one of the most promising host materials for the intercalation of multivalent ions. Here, we report on the fabrication of a binder-free and self-supporting V2O5 micrometer-thick paper-like electrode material and its use as the cathode for rechargeable aluminum-ion batteries. The electrical conductivity of the cathode was significantly improved by a novel in-situ and self-limiting copper migration approach into the V2O5 structure. This process takes advantage of the dissolution of Cu by the ionic liquid-based electrolyte, as well as the presence of two different accommodation sites in the nanostructured V2O5 available for aluminum-ions and the migrated Cu. Furthermore, the advanced nanostructured cathode delivered a specific discharge capacity of up to ~170 mAh g-1 and the reversible intercalation of Al3+ for more than 500 cycles with a high Coulomb efficiency reaching nearly 100%. The binder-free concept results in an energy density of 74 Wh kg-1, which shows improved energy density in comparison to the so far published V2O5-based cathodes. Our results provide valuable insights for the future design and development of novel binder-free and self-supporting electrodes for rechargeable multivalent metal-ion batteries associating a high energy density, cycling stability, safety and low cost.
Keywords: V2O5 cathode; aluminum-ion battery; binder-free electrode; paper-like thin films; post-lithium-ion batteries.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Moretti A., Passerini S. Bilayered Nanostructured V2O5·nH2O for Metal Batteries. Adv. Energy Mater. 2016;6:1600868. doi: 10.1002/aenm.201600868. - DOI
-
- Yao J., Li Y., Massé R.C., Uchaker E., Cao G. Revitalized interest in vanadium pentoxide as cathode material for lithium-ion batteries and beyond. Energy Storage Mater. 2018;11:205–259. doi: 10.1016/j.ensm.2017.10.014. - DOI
-
- Etacheri V., Marom R., Elazari R., Salitra G., Aurbach D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011;4:3243–3262. doi: 10.1039/c1ee01598b. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

