Synthesis of 2,2,6-Trisubstituted 5-Methylidene-tetrahydropyran-4-ones with Anticancer Activity
- PMID: 32019209
- PMCID: PMC7038078
- DOI: 10.3390/molecules25030611
Synthesis of 2,2,6-Trisubstituted 5-Methylidene-tetrahydropyran-4-ones with Anticancer Activity
Abstract
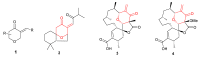
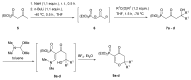
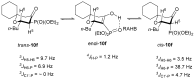
In our continuous search for new, relatively simple 2-alkylidene-1-oxoheterocycles as promising anticancer drug candidates, herein we report an efficient synthesis of 2,2,6-trisubstituted 5-methylidenetetrahydropyran-4-ones. These compounds were obtained in a four step reaction sequence, in which starting diethyl 2-oxopropylphosphonate was transformed into 2,2-disubstituted 5-diethoxyphosphoryldihydropyran-4-ones, followed by Michael addition of various Grignard reagents and Horner-Wadsworth-Emmons reaction of the obtained adducts with formaldehyde. Stereochemistry of the intermediate Michael adducts is also discussed. Final 5-methylidenetetrahydropyran-4-ones were tested for their possible antiproliferative effect against three cancer cell lines and 6-isopropyl-2,2-dimethyl-5-methylidenetetrahydropyran-4-one (11c), which showed very high cytotoxic activity against HL-60 human leukemia cells and was three times more active than known anticancer drug carboplatin, was selected for further biological evaluation, in order to disclose its mechanism of action. The obtained results indicated that 11c induced apoptosis in HL-60 cells and caused the arrest of the cell cycle in the G2/M phase, which may suggest its cytotoxic and cytostatic activity.
Keywords: Horner-Wadsworth-Emmons olefination; Michael addition; apoptosis; cell cycle; cytotoxic activity; methylidenedihydropyran-4-ones.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
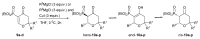
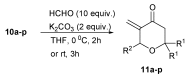


Similar articles
-
Stereoselective Synthesis and Anticancer Activity of 2,6-Disubstituted trans-3-Methylidenetetrahydropyran-4-ones.Materials (Basel). 2022 Apr 21;15(9):3030. doi: 10.3390/ma15093030. Materials (Basel). 2022. PMID: 35591364 Free PMC article.
-
Synthesis and Cytotoxic Evaluation of 3-Methylidenechroman-4-ones.Molecules. 2019 May 15;24(10):1868. doi: 10.3390/molecules24101868. Molecules. 2019. PMID: 31096601 Free PMC article.
-
4-Methylideneisoxazolidin-5-ones--a new class of alpha-methylidene-gamma-lactones with high cytostatic activity.Bioorg Med Chem Lett. 2006 Mar 1;16(5):1430-3. doi: 10.1016/j.bmcl.2005.11.032. Epub 2005 Nov 28. Bioorg Med Chem Lett. 2006. PMID: 16314098
-
Synthesis of 4,4-Disubstituted 3-Methylidenechroman-2-ones as Potent Anticancer Agents.ChemMedChem. 2017 Apr 20;12(8):599-605. doi: 10.1002/cmdc.201700080. Epub 2017 Mar 22. ChemMedChem. 2017. PMID: 28258688
-
Synthesis of 3-Methylidene-1-tosyl-2,3-dihydroquinolin-4(1H)-ones as Potent Cytotoxic Agents.Chem Biodivers. 2018 Sep;15(9):e1800242. doi: 10.1002/cbdv.201800242. Epub 2018 Aug 8. Chem Biodivers. 2018. PMID: 29935105
Cited by
-
Stereoselective Synthesis and Anticancer Activity of 2,6-Disubstituted trans-3-Methylidenetetrahydropyran-4-ones.Materials (Basel). 2022 Apr 21;15(9):3030. doi: 10.3390/ma15093030. Materials (Basel). 2022. PMID: 35591364 Free PMC article.
References
-
- Pięta M., Kędzia J., Kowalczyk D., Wojciechowski J., Wolf W.M., Janecki T. Enantioselective synthesis of 5-methylidenedihydrouracils as potential anticancer agents. Tetrahedron. 2019;75:2495–2505. doi: 10.1016/j.tet.2019.03.024. - DOI
-
- Jiang Z.-Y., Zhou J., Huang C.-G., Hu Q.-F., Huang X.-Z., Wang W., Zhang L.-Z., Li G.-P., Xia F.-T. Two novel antiviral terpenoids from the cultured Perovskia atriplicifolia. Tetrahedron. 2015;71:3844–3849. doi: 10.1016/j.tet.2015.04.017. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

