Polyethylene Glycol 35 (PEG35) Protects against Inflammation in Experimental Acute Necrotizing Pancreatitis and Associated Lung Injury
- PMID: 32019239
- PMCID: PMC7036920
- DOI: 10.3390/ijms21030917
Polyethylene Glycol 35 (PEG35) Protects against Inflammation in Experimental Acute Necrotizing Pancreatitis and Associated Lung Injury
Abstract
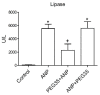
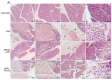
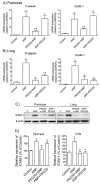
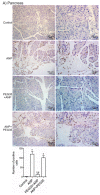
Acute pancreatitis is an inflammatory disorder of the pancreas. Its presentation ranges from self-limiting disease to acute necrotizing pancreatitis (ANP) with multiorgan failure and a high mortality. Polyethylene glycols (PEGs) are non-immunogenic, non-toxic, and water-soluble chemicals composed of repeating units of ethylene glycol. The present article explores the effect of PEG35 administration on reducing the severity of ANP and associated lung injury. ANP was induced by injection of 5% sodium taurocholate into the biliopancreatic duct. PEG35 was administered intravenously either prophylactically or therapeutically. Three hours after ANP induction, pancreas and lung tissue samples and blood were collected and ANP severity was assessed. To evaluate the inflammatory response, gene expression of pro-inflammatory cytokines and chemokine and the changes in the presence of myeloperoxidase and adhesion molecule levels were determined in both the pancreas and the lung. To evaluate cell death, lactate dehydrogenase (LDH) activity and apoptotic cleaved caspase-3 localization were determined in plasma and in both the pancreatic and lung tissue respectively. ANP-associated local and systemic inflammatory processes were reduced when PEG35 was administered prophylactically. PEG35 pre-treatment also protected against acute pancreatitis-associated cell death. Notably, the therapeutic administration of PEG35 significantly decreased associated lung injury, even when the pancreatic lesion was equivalent to that in the untreated ANP-induced group. Our results support a protective role of PEG35 against the ANP-associated inflammatory process and identify PEG35 as a promising tool for the treatment of the potentially lethal complications of the disease.
Keywords: cytokines; inflammation; pancreatitis; polyethylene glycols; pulmonary injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



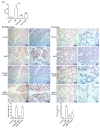
References
-
- Banks P.A., Bollen T.L., Dervenis C., Gooszen H.G., Johnson C.D., Sarr M.G., Tsiotos G.G., Vege S.S. Acute Pancreatitis Classification Working G: Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. - DOI - PubMed
-
- Ivens I.A., Achanzar W., Baumann A., Brändli-Baiocco A., Cavagnaro J., Dempster M., Depelchin B.O., Rovira A.R., Dill-Morton L., Lane J.H., et al. PEGylated Biopharmaceuticals: Current Experience and Considerations for Nonclinical Development. Toxicol. Pathol. 2015;43:959–983. doi: 10.1177/0192623315591171. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

