Fluid and solute transport across the retinal pigment epithelium: a theoretical model
- PMID: 32019471
- PMCID: PMC7061709
- DOI: 10.1098/rsif.2019.0735
Fluid and solute transport across the retinal pigment epithelium: a theoretical model
Abstract
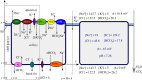
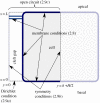
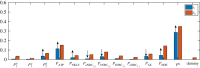
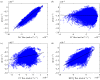
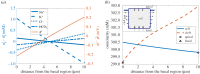
The retina is composed of two main layers-the neuroretina and the retinal pigment epithelium (RPE)-that are separated by a potential gap termed the sub-retinal space (SRS). Accumulation of fluid in the SRS may result in a retinal detachment. A key function of the RPE is to prevent fluid accumulation in the SRS by actively pumping fluid from this space to the choroid. We have developed a mathematical model of this process that incorporates the transport of seven chemical species: Na+, K+, Cl-, , H+, CO2 and H2CO3. This allows us to estimate solute and water fluxes and to understand the role of the different membrane ion channels. We have performed a global sensitivity analysis using the extended Fourier amplitude sensitivity test to investigate the relative importance of parameters in generating the model outputs. The model predicts that flow across the RPE is driven by an osmotic gradient in the cleft gap between adjacent cells. Moreover, the model estimates how water flux is modified in response to inhibition of membrane ion channels and carbonic anhydrase (CA). It provides a possible explanation for how CA inhibitors, which are used clinically to prevent fluid accumulation in the SRS, may be acting.
Keywords: fluid transport across epithelia; ion transport; retinal pigment epithelium.
Conflict of interest statement
We declare we have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
