Effects of low versus standard pressure pneumoperitoneum on renal syndecan-1 shedding and VEGF receptor-2 expression in living-donor nephrectomy: a randomized controlled study
- PMID: 32019488
- PMCID: PMC7001365
- DOI: 10.1186/s12871-020-0956-7
Effects of low versus standard pressure pneumoperitoneum on renal syndecan-1 shedding and VEGF receptor-2 expression in living-donor nephrectomy: a randomized controlled study
Abstract
Background: Laparoscopic nephrectomy is a preferred technique for living kidney donation. However, positive-pressure pneumoperitoneum may have an unfavorable effect on the remaining kidney and other distant organs due to inflamed vascular endothelium and renal tubular cell injury in response to increased systemic inflammation. Early detection of vascular endothelial and renal tubular response is needed to prevent further kidney injury due to increased intraabdominal pressure induced by pneumoperitoneum. Transperitoneal laparoscopic living donor nephrectomy represented a human model of mild increasing intraabdominal pressure. This study aimed to assess the effect of increased intraabdominal pressure on vascular endothelium and renal tubular cells by comparing the effects of low and standard pressure pneumoperitoneum on vascular endothelial growth factor receptor-2 (VEGFR-2) expression and the shedding of syndecan-1 as the early markers to a systemic inflammation.
Methods: We conducted a prospective randomized study on 44 patients undergoing laparoscopic donor nephrectomy. Subjects were assigned to standard (12 mmHg) or low pressure (8 mmHg) groups. Baseline, intraoperative, and postoperative plasma interleukin-6, syndecan-1, and sVEGFR-2 were quantified by ELISA. Syndecan-1 and VEGFR-2 expression were assessed immunohistochemically in renal cortex tissue. Renal tubule and peritubular capillary ultrastructures were examined using electron microscopy. Perioperative hemodynamic changes, end-tidal CO2, serum creatinine, blood urea nitrogen, and urinary KIM-1 were recorded.
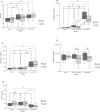
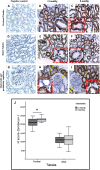
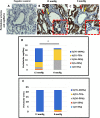
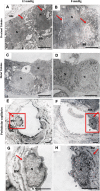
Results: The low pressure group showed lower intra- and postoperative heart rate, intraoperative plasma IL-6, sVEGFR-2 levels and plasma syndecan-1 than standard pressure group. Proximal tubule syndecan-1 expression was higher in the low pressure group. Proximal-distal tubules and peritubular capillary endothelium VEGFR-2 expression were lower in low pressure group. The low pressure group showed renal tubule and peritubular capillary ultrastructure with intact cell membranes, clear cell boundaries, and intact brush borders, while standard pressure group showed swollen nuclei, tenuous cell membrane, distant boundaries, vacuolizations, and detached brush borders.
Conclusion: The low pressure pneumoperitoneum attenuated the inflammatory response and resulted in reduction of syndecan-1 shedding and VEGFR-2 expression as the renal tubular and vascular endothelial proinflammatory markers to injury due to a systemic inflammation in laparoscopic nephrectomy.
Trial registration: ClinicalTrials.gov NCT:03219398, prospectively registered on July 17th, 2017.
Keywords: Interleukin-6; Laparoscopic nephrectomy; Pneumoperitoneum; Renal resistive index; Syndecan-1; sVEGFR-2.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Smit M, Hofker HS, Leuvenink HG, Krikke C, Jongman RM, Zijlstra JG, van Meurs M. A human model of intra-abdominal hypertension: even slightly elevated pressure lead to increased acute systemic inflammation and signs of acute kidney injury. Crit Care. 2013;17(2):425. doi: 10.1186/cc12568. - DOI - PMC - PubMed
-
- Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the world Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39(7):1190–1206. doi: 10.1007/s00134-013-2906-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

