Prevention of doxorubicin-induced Cardiotoxicity by pharmacological non-hypoxic myocardial preconditioning based on Docosahexaenoic Acid (DHA) and carvedilol direct antioxidant effects: study protocol for a pilot, randomized, double-blind, controlled trial (CarDHA trial)
- PMID: 32019575
- PMCID: PMC7001267
- DOI: 10.1186/s13063-019-3963-6
Prevention of doxorubicin-induced Cardiotoxicity by pharmacological non-hypoxic myocardial preconditioning based on Docosahexaenoic Acid (DHA) and carvedilol direct antioxidant effects: study protocol for a pilot, randomized, double-blind, controlled trial (CarDHA trial)
Abstract
Background: Anthracycline-induced cardiotoxicity (AIC), a condition associated with multiple mechanisms of damage, including oxidative stress, has been associated with poor clinical outcomes. Carvedilol, a β-blocker with unique antioxidant properties, emerged as a strategy to prevent AIC, but recent trials question its effectiveness. Some evidence suggests that the antioxidant, not the β-blocker effect, could prevent related cardiotoxicity. However, carvedilol's antioxidant effects are probably not enough to prevent cardiotoxicity manifestations in certain cases. We hypothesize that breast cancer patients taking carvedilol as well as a non-hypoxic myocardial preconditioning based on docosahexaenoic acid (DHA), an enhancer of cardiac endogenous antioxidant capacity, will develop less subclinical cardiotoxicity manifestations than patients randomized to double placebo.
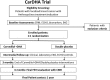
Methods/design: We designed a pilot, randomized controlled, two-arm clinical trial with 32 patients to evaluate the effects of non-hypoxic cardiac preconditioning (DHA) plus carvedilol on subclinical cardiotoxicity in breast cancer patients undergoing anthracycline treatment. The trial includes four co-primary endpoints: changes in left ventricular ejection fraction (LVEF) determined by cardiac magnetic resonance (CMR); changes in global longitudinal strain (GLS) determined by two-dimensional echocardiography (ECHO); elevation in serum biomarkers (hs-cTnT and NT-ProBNP); and one electrocardiographic variable (QTc interval). Secondary endpoints include other imaging, biomarkers and the occurrence of major adverse cardiac events during follow-up. The enrollment and follow-up for clinical outcomes is ongoing.
Discussion: We expect a group of anthracycline-treated breast cancer patients exposed to carvedilol and non-hypoxic myocardial preconditioning with DHA to show less subclinical cardiotoxicity manifestations than a comparable group exposed to placebo.
Trial registration: ISRCTN registry, ID: ISRCTN69560410. Registered on 8 June 2016.
Keywords: Anthracyclines; CarDHA; Carvedilol; Chemotherapy-induced cardiotoxicity; DHA; Study protocol.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, ESC Scientific Document Group et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(36):2768–2801. doi: 10.1093/eurheartj/ehw211. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

