Pre-clinical characterisation of E2814, a high-affinity antibody targeting the microtubule-binding repeat domain of tau for passive immunotherapy in Alzheimer's disease
- PMID: 32019610
- PMCID: PMC7001291
- DOI: 10.1186/s40478-020-0884-2
Pre-clinical characterisation of E2814, a high-affinity antibody targeting the microtubule-binding repeat domain of tau for passive immunotherapy in Alzheimer's disease
Abstract
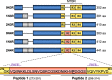
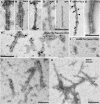
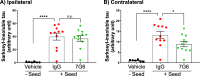
Tau deposition in the brain is a pathological hallmark of many neurodegenerative disorders, including Alzheimer's disease (AD). During the course of these tauopathies, tau spreads throughout the brain via synaptically-connected pathways. Such propagation of pathology is thought to be mediated by tau species ("seeds") containing the microtubule binding region (MTBR) composed of either three repeat (3R) or four repeat (4R) isoforms. The tau MTBR also forms the core of the neuropathological filaments identified in AD brain and other tauopathies. Multiple approaches are being taken to limit tau pathology, including immunotherapy with anti-tau antibodies. Given its key structural role within fibrils, specifically targetting the MTBR with a therapeutic antibody to inhibit tau seeding and aggregation may be a promising strategy to provide disease-modifying treatment for AD and other tauopathies. Therefore, a monoclonal antibody generating campaign was initiated with focus on the MTBR. Herein we describe the pre-clinical generation and characterisation of E2814, a humanised, high affinity, IgG1 antibody recognising the tau MTBR. E2814 and its murine precursor, 7G6, as revealed by epitope mapping, are antibodies bi-epitopic for 4R and mono-epitopic for 3R tau isoforms because they bind to sequence motif HVPGG. Functionally, both antibodies inhibited tau aggregation in vitro. They also immunodepleted a variety of MTBR-containing tau protein species. In an in vivo model of tau seeding and transmission, attenuation of deposition of sarkosyl-insoluble tau in brain could also be observed in response to antibody treatment. In AD brain, E2814 bound different types of tau filaments as shown by immunogold labelling and recognised pathological tau structures by immunohistochemical staining. Tau fragments containing HVPGG epitopes were also found to be elevated in AD brain compared to PSP or control. Taken together, the data reported here have led to E2814 being proposed for clinical development.
Keywords: Alzheimer; Tau; immunotherapy; neurodegeneration; tauopathy.
Conflict of interest statement
All work performed to generate data reported in this manuscript was funded by Eisai, a pharmaceutical company listed on the Tokyo Stock Exchange (TYO:4523). MR, YI, KM, ST, MD, JG, HO, ZZ, SA, NT, MO, MA, HA, KLA, JS, EA, KH and JMS were full-time employees of Eisai for the period in which data reported in this study were generated. IS and ES are UCL employees funded by Eisai through the Eisai:UCL Therapeutic Innovation Group (TIG). The TIG is a collaborative partnership between Eisai and UCL to discover and advance medical therapies for neurodegenerative disorders. Successful medical therapies from the collaboration could potentially be of commercial benefit to both Eisai and UCL. RdS and KS are UCL employees and also funded by the Reta Lila Weston Trust for Medical Research.
Figures







References
-
- Ahmed Z, Cooper J, Murray TK, Garn K, McNaughton E, Clarke H, Parhizkar S, Ward MA, Cavallini A, Jackson S, et al. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 2014;127:667–683. doi: 10.1007/s00401-014-1254-6. - DOI - PMC - PubMed
-
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. - DOI - PMC - PubMed
-
- Arai T, Ikeda K, Akiyama H, Nonaka T, Hasegawa M, Ishiguro K, Iritani S, Tsuchiya K, Iseki E, Yagishita S, et al. Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol. 2004;55:72–79. doi: 10.1002/ana.10793. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

